| Potassium-Air Batteries |
The potassium-air
battery is an interesting technology, and a company, Kair, was founded in 2014
to commercialize it. It was founded by a student team at Ohio State University,
and started producing an impressive amount of hype. They proceeded to win a
boat load of business plan competitions and collapsed in a heap two years
later. It looks like they spent all their time and money entering competitions,
not much on product development.
However, it looks like there are still
some major problems. In 2021 a paper was published "The Potassium–Air
Battery: Far from a Practical Reality?" and in 2022
https://www.researchgate.net/publication/361024916_Advances_challenges_and_environmental_impacts_in_metal-air_battery_electrolytes
which points out the problems with potassium-air cells is dendrite formation
when plating the potassium metal during charging and water vapor from the air
corroding the anode.
Very difficult problems for a startup company run by
students. |
| Linear non-threshold debunked
again |
The radiation
regulatory environment has long be dominated by the idea that any radiation is
bad news. However, the evidence has been rolling out for years that this is not
the case. Among many examples is the fact that there are places in Iran and
China that have background radiation levels orders of magnitude higher than the
regulatory limits without bad things happening to the population, either
newcomers or those that have lived there many generations.
http://ww.nuceng.ca/refer/radiation/Ramsar.pdf
And
the study of 20,000 Hiroshima and Nagasaki survivors, the most studied group in
history, have had extensive medical testing and have been followed closely
without any sign of higher levels of health problems. In fact they have been
healthier than the general Japanese population.
https://inis.iaea.org/search/search.aspx?orig_q=RN:32005454
A
new study has shown that the man who got the Nobel prize for radiation mutation
experiments and was the creator of the linear-no-threshold model faked his
data.
https://peh-med.biomedcentral.com/articles/10.1186/s13010-018-0066-z
This
can be technical enough to bog you down, so a useful summary is here
https://townhall.com/columnists/pauldriessen/2018/11/08/fraudulent-science-behind-radiation-regulations-n2535499
This
fear of radiation has not only wasted billions of dollars and untold wasted
man-hours, but has resulted in thousands of unfortunate elective abortions in
Europe after the Chernoble disaster. Is it possible to reverse the damage and
culture-of-fear generated by this evil scientist? |
| LED Lies? |
I love LEDs. I met
Nick Holonyak, the inventer of the LED years ago. But they are not magic. We
are continuously receiving news snippets about how much money municipalities
are saving by switching over to LED street lighting. The Department of Energy
has an interest in LED lighting, and have published this report
https://www.energy.gov/eere/ssl/led-basics
Under
the category "How efficient are LEDs?" it shows that the luminous efficiency of
the highest efficiency LED fixtures is 109 lumens per watt, the efficiency of
linear fluorescents is 108 lumens per watt, high intensity discharge lamp
efficiency is 115 lumens per watt, and low wattage high intensity discharge
lamps efficiency is 104 lumens per watt.
So where is the "50-75% less
energy" and "$700,000 a year" in savings that Bakersfield is claiming?
https://iconsofinfrastructure.com/bakersfield-upgrades-to-led-street-lamps/
Part
of it could be the labor to replace bad bulbs. But, as is often the case,
someone is lying, I'm not sure why. It turns out that metal vapor lamps are
very very efficient, as are fluorescent lamps. LEDs have their place,
particularly where single-color light is needed, but they shouldn't be
over-hyped, and they shouldn't be replaced by taxpayers that are already being
stretched. |
Best of show at CES 2018
 |
PowerStream's industrial design
division Raptor Creative designed the Origin PC Millennium, which one
HotHardware's Best of CES 2018 award.

This computer
has also won an Editor's Choice award from Digital Trends |
| Q: Organic food? |
It is a huge marketing success, a
societal failure. You don't get something for nothing, what you get is food
that is not superior in any way, but consumes more of the earth's precious
resources. At what price smugness?
https://www.prageru.com/videos/organic-food-worth-cost |
| Q: Unique Motor Uses Only Permanent
Magnets – No Electric Power Required. --??? |
No link will be posted, we don't
want to associate our good name with this mess. But a quick search will find an
article at Power Electronics.
This invention claims to use permanent
magnets to generate free energy.
This is a scam.
Magnetic
forces are fundamentally and essentially conservative: it will always take as
much energy to separate them as was extracted putting them together. No matter
how many fancy configurations and bogus charts an inventer assembles, the
bottom line is that if there were non-conservative forces available they would
have shown up in many obvious ways before now. But even the most subtle
experiments have failed to detect anything but conservative magnetic forces.
Notwithstanding the inventor's references to the journals Nature, and Physical
Review, and physicists Roger Penrose and Richard Feynman!
If it seems
too good to be true, hang on to your wallet with both hands!
|
| Q: Lead acid battery voltage |
I bought your PST-3P10-12VA with
alligator clips and use it to charge for all my BP7-12 B&B SLA batteries. I
use these batteries at the RC fields for RC car starter boxes and gasoline or
nitromethane fuel pumps. Their use is minimal with maybe 5-10 seconds bursts on
the starter boxes which use 540 brushed motors and 45-60 seconds for my
gasoline or nitromethane fuel pump. I always bring two batteries to the field
and half day, I switch over as to not over-discharge the batteries. After every
day at the field, I use the charger mentioned herein to recharge the batteries.
However, I now notice that after charging is complete and when I remove the
charger, the voltage on almost all the batteries I have, 8 in total, in about
1-3 days drops and holds at approx. 12.2V to 12.9V on almost all the batteries.
Is this normal? I thought 13.6V should be the min. I do have two batteries
which hold at 13.22V-13.33V.
A: The chemistry voltage of a 12V lead acid
battery is 12.9V. They are charged at higher voltages in order to get a faster
charge, as long as the voltage is below about 14V the battery will not
overcharge. The PST-3P10-12VA floats the battery at a safe 13.8V. When the
charger is removed the battery has excess charge on the plates, they act like a
capacitor. After this excess charge dissipates the fully charged battery should
be at about 12.9V. I don't know why some of your batteries show as low as
12.2V. |
| Idiegogo project using one of
PowerStream's ultra-small lithium polymer cells |
https://www.indiegogo.com/projects/mu-tag-world-s-smallest-loss-prevention-device--5#/ |
| Q: Inverters and refrigerators |
Q: I just read your informative FAQ
article on inverters. I was an electronics technician in a previous life but am
struggling to understand why a refrigerator I'm considering for my boat has a
warning that it should not be used with an inverter. If it's a pure sine wave
inverter with sufficient capacity, what could the problem be? I don't
understand how the fridge would see any difference from being plugged in to a
standard 120V outlet. --N
A: Some problems that I can think of is that
(as you point out) a modified sine wave inverter will heat up the motor because
the 35% that isn't sine wave gets turned to heat in the motor. And of course
you need a lot more inverter power to overcome that deficit and the fact that
inverter manufacturers exaggerate.
Secondly, the inverter manufacturer
that we represent won't sell an inverter into a compressor application like
refrigeration unless they know the locked rotor current. Compressors take a lot
more current to start than to run, and the inverter has to be rated to supply
continuous power at the locked rotor current in order to be reliable. |
| Q: Flashlight running on body
heat? |
A recent Kickstarter project has
raised $80,000 as of late October to develop a flashlight that runs on body
heat. I am so skeptical of this that I am going to come down on the side of
"intentional scam." Thermoelectric generators are fundamentally low efficiency.
The best thermoelectric generators now in existence have a ZT parameter of 0.9
to 1.0, or about 1/8 the efficiency of a perfect heat engine.
The
theoretical efficiency of a perfect heat engine is [1 - Tcold/Thot] if the temperature is in
kelvins. If the hot side is normal body temperature of 307 kelvins (skin
temperature is usually about 307), and the cold side room temperature of 297
kelvins (75 degrees F), then the theoretical maximum efficiency is 3.2%. So 1/8
of 3.2% is 0.4%.
In other words, in order to generate 1 watt of useful
power, you have to move 250 watts of heat. It takes 2 to 5 watts minimum to
charge your cell phone, so you would have to move 500 to 1250 watts of heat to
charge your cell phone.
The inventor intends to drive a 3000 mCD Cree
LED, possibly the one that requires 3.2V at 20mA = 64 mW. So you would have to
move 16 watts of heat to power this flashlight, while keeping your hot thumb
and the cold heat sink at constant temperatures of 98.6°F and 77°F
respectively. A typical human at rest puts out about 100 watts over the whole
body. So at most you should be able to get a few seconds of light before your
hand cools down. If you just use your thumb as the Kickstarter project shows,
your thumb would cool at about 1 degree Celsius per second, halving the
available power in 5 seconds.
Similarly getting a heat sink to dissipate 16
watts with a temperature difference to the air of only a degree or two will
require a lot of aluminum or a lot of air motion.
Lastly, his headline
is "Have you ever thought how much energy is wasted by humanity?"
It turns
out that my energy is used to keep my fingers warm, not wasted at all. |
| Q: I need 24 volts in a PC system,
is it possible to modify an ATX power supply to also supply 24V? |
A. Not directly, since all the 12V
lines are in parallel, and the -12V line in series with the +12V line gives
little power. You can use a DC/DC
converter to conveniently boost the 12V (or 5V or 3.3V) to 24V. |
| Q: What do you think about
Batteriser? |
A. I have always had in the back of
my to-do list a project to make a web page that tracks press releases on
battery technologies and show that 99.99% never make it to the market. The
Batteriser is sure to be on that list. It has an enormous amount of hype, and
lot of it is obviously a fraud. For example, "Someone broke in and stole all my
prototypes." or "A completely new alkaline battery is rated to generate 1.5
volts, but once its output drops below 1.35 or even 1.4 volts, it effectively
becomes useless to many devices." The truth is that alkaline batteries have
1.5V as their peak voltage, and quickly drop below 1.4 volts as soon as you
start using them.
The biggest clue is that they aren't going to be
available until September, so why generate all they hype now? I don't doubt for
a moment that he can make a little DC/DC converter that will boost the voltage
to 1.5V. But this will subtract from the total energy in the battery because of
its inefficiency.
I really like his deceptive demo. He took a couple of
batteries and put them in a "power meter," they read 1.3V and announced to the
journalists that this means they were dead. (Not dead, but probably had 80% or
more of their capacity remaining, see our discharge curves at
/AA-tests.htm). Then he applied the voltage
booster, which of course showed that the voltage went up to 1.5V as if by
magic. Then he put them in a bluetooth keyboard and the keyboard showed that
the remaining capacity is 100%. Of course the keyboard doesn't have mystical
powers of perception, it sees the 1.5V and assumes that fresh batteries were
placed in it, but was fooled by the voltage. How long the batteries will last
in such a configuration is not demonstrated at all.
AND he says that
this is a great way to save the planet from batteries in landfills. Of course
there is nothing hazardous to the environment in an alkaline cell.
AND
one of my favorite ploys used by technology scammers is used. He says "Big
Battery Doesn't Understand Electronics." |
| Q: I have a switchmode power supply
that is working fine but it smells bad because of overheating. I thought these
were protected from overcurrent. |
A: A little education about
switchmode power supplies may be in order. They are all designed to run at
110-120% of rated power without shutting down so that they can handle surge
currents and short overcurrents without giving you grief.
At powers
above this they will try to protect themselves.
So there is a
no-mans-land of 100% to 120% rated power in which they will look like they are
working fine, but they are really overheating and will fail prematurely if run
continuously. Add this to the fact that many low-cost manufacturers lie about
the power supply's capability and you might get a little extra smoke. |
| Quality versus Price |
Dear PowerStream,
I've been
researching the reliability of small 5V "wall wart" type power supplies for USB
and other applications. In the process I was searching for wire current
carrying capacity and found your "Wire Gauge and Current Limits" page. What a
helpful page, thanks!
After perusing that I looked around your site
further. I have a couple of comments which will no doubt >not< be news to
you. These things (small 5V supplies) can be found on Ebay or Amazon very
cheaply, and comparatively make your offerings seem expensive. For instance I
just bought a product similar to your model PST-AC0520W for $5.29, delivered to
my home from a US supplier!
But the reliability of these cheapo supplies is
notoriously bad, which is NOT apparent to non-technical buyers. They are known
to use electrolytic capacitors which are underrated for the application's
ripple current. The capacitors get hot, go bad, and cause the power supplies to
have a short lifetime.
The other thing that happens is that the
manufacturers out-and-out LIE about the specifications. My cheapo unit is
labeled "5V, 2A," but shuts down if you try to draw more than 1.4A from it. And
its output cord has undersized 24 AWG wires in it (which led me to your site).
So all of this long-winded discussion leads to a suggestion: on your
products' web pages compare the performance and reliability of the cheapo units
to yours, and make it clear to potential buyers that they are well worth the
few extra dollars. Many many non-technical people have been screwed by your
unscrupulous competition.
Regards, Curt
A: Thanks for your analysis,
Curt. We try to sort the good from bad suppliers for our customers. It might be
fun to make a "tear down" video comparing cheap versus quality power supplies.
I'll put it on my to-do list.
--mark |
 A KickStarter project worth supporting A KickStarter project worth supporting |
PowerStream has had fun supporting
the SAM project with batteries and advice. You are going to want one of these
kits of internet connected gadgets that can be joined wirelessly into any
gadget you can conceive. The funding ends on about 29 October 2014
https://www.kickstarter.com/projects/1842650056/sam-the-ultimate-internet-connected-electronics-ki
Update:
Sam Labs has been successful in turning its Kickstarter fund raising into a
successsful business. Check them out at http://www.samlabs.me |
| Low temperature batteries |
Q: Hi Powerstream people, I have a
customer looking for a battery solution for an application in Canada that could
get to -20C in the winter. He wants it to solar charge but be able to go at
least a week without charging. Is there a lithium-ion battery pack that could
work? Thanks, Ed
A: Hi Ed, There is a fundamental problem, lithium ion
batteries cannot be charged at less than zero degrees C (some say -10C). At low
temperatures the crystal channels get clogged by lithium metal and it kills the
batteries.
The best battery for this application is an a appropriately
sized sealed lead acid battery. The sulfuric acid in a fully charged battery
freezes at about -72 C, and for a half charged battery at about -17C. So if you
keep the charge level above 60% the freezing temperature is below about -24 C.
As you can calculate yourself, 7 amp hours divided by .6 is 11.7AH, so anything
bigger than that is safe as long as the solar panel can recharge it. |
| Charging a lithium ion cell with a
solar cell |
Q: Hi, I have been reading up on
lipo charging.
From what I gather, I see it is charged at a constant
current then at some point a constant voltage is put on and the charging is cut
off when the current has diminished to about 5% c. ( depending ).
What
if I don't mind never fully charging the cell. I directly put on a solar cell
array which can not provide more than 1C current and can never push a higher
potential than 4.05 volts. Surely this will charge the cell correct ? Slow, but
surely ? Or is there some essential reason why a constant current should be
provided at the start of the charging sequence ? -Albert.
A: Hi Albert,
Yes, you can do that and it will work well. But you will have to live with the
fact that the battery will never get to full charge. Here is the explanation
including original research we did on the subject
/lithuim-ion-charge-voltage.htm
The "constant current" means that the
charger can't put out more than that current, so the voltage is lowered to make
sure that no more is drawn. As the battery fills up the voltage raises until it
gets to the maximum voltage the battery can take, and then holds at that
voltage while the current drops.
best regards
mark |
| Charging lithium ion cells (and
lithium polymer cells) in parallel |
Q: I have a question about putting
two Li-Poly batteries in parallel. I have a 33 x 27 x 8mm space for as much
battery capacity as I can get, and stacking two GM382530-PCB batteries in
parallel would be perfect. Besides needing to bring them to same potential
before initially connecting them in parallel, is there any danger or does it
harm the batteries by charging and discharging them while connected in
parallel? Thanks! Matt
A: Lithium batteries are unusual in that they
like to be charged in parallel, and they don't even have to be at the same
charge state or even the same size cell. They are very picky about being put in
series, but parallel is OK. |
| A very audacious fake Ultrafire
lithium ion 18650 |
Here is the youtube video, but the
executive summary is that the manufacturer put a 66maH electric cigarette
battery in an 18650 package, and filled the rest of the cell with
flour.
 https://www.youtube.com/watch?feature=player_embedded&v=eOshOXcSkDA
https://www.youtube.com/watch?feature=player_embedded&v=eOshOXcSkDA
|
The difference between a battery charger and a power
supply. |
There is a problem that a battery
charger has to solve that a power supply does not. When you apply 4.6 volts to
a 4V lead acid battery it is impossible to know how much current the battery
will suck up. A 1000AH battery one would probably take 100s of amps if it was
fully discharged. So a battery charger has a "constant current" circuit that
cuts back the voltage until the battery is asking for exactly what the charger
wants to supply. Then, as the battery fills up, that voltage is increased so
that the current stays constant. Eventually the voltage will top out and then
the current slowly drops as the battery fills up. Some benchtop power supplies
have the constant current limit feature, and if you are trying to charge an
odd-voltage lead acid or lithium battery they are the simplest
alternative. |
Another bad idea for energy harvesting
  |
Here is another one from the same source. The
idea is to put a couple of scoops in front of the electric or hybrid car to run
a generator to charge the battery. Ignoring the fact that squirrel cage fans
work on centrifugal force and therefore don't run backwards by pushing air at
them, this is another example of putting more load on the car's motor by adding
extra drag on the car. Therefore you are going to lose energy rather than
harvest some wasted energy. |
A bad idea for energy
harvesting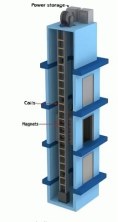 |
We get passed a lot of interesting ideas for
alternative or free energy generation. I thought about creating a web page
based on these, showing the fallacies, but of course most of these are closely
held secrets and the inventor would be upset and probably litigious if we
divulged them.
However, I just got an e-mail from an invention promotion
company that has public links, so I can discuss it. The invention can be found
here http://www.sellidea.com/?id=6239
Here is why this is a bad idea. It is usually useful to ask "where is the
energy coming from?" In this case he proposes a generator analogous to a linear
motor to be run along elevator or conveyer belts to extract energy in the form
of electricity. The conveyer belt is the easiest to debunk. Obviously, the only
source of energy in the conveyer belt is the motor that drives it. Any energy
extracted from the belt's motion will add to the motor's load, and therefore
cost more energy than is generated.
The elevator has to deal with
gravity, however, which is more mysterious. There are counter-weights in the
elevator that compensate for the weight of the elevator and cables, so for an
empty elevator the motor is just overcoming friction, no actual potential
energy is being generated when the elevator moves to the top floor. (I know
that this is a simplification of what must be a lot of counterweight
engineering, but it is true enough). When a load is added to the elevator,
there is potential energy stored as the elevator moves up, and lost as the
elevator moves down. If there is energy to be extracted from the downward
motion, the motor is still in the circuit, and could be used for that purpose
if practical. There is no energy available for extraction as the elevator moves
up, since any generator added will add to the load of the motor, consuming more
energy.
|
| Mystery Battery |
An antique 6V
lead acid battery was recently unearthed in Washington State. The finder is
looking for more information about this. Take a look. |
| Shock voltage |
Q: Hello, I was just reading how to design
battery packs on your website, and I just have a few questions. I'm wanting to
build some 7.2volt Nicd packs for RC cars. I've read how to do it, but was just
wondering if I could get shocked while doing it? I read that you can short the
battery if you touch the + and - wires together, but what if you just held in
each hand the positive and negative wires at the same time? Would that complete
the circuit and I get shocked? I'm asking because with rc packs, there really
isn't any insulation for the battery tabs, because we use discharge trays in
racing to always keep the cells matched, and the trays have to touch each cell
tab.
A: Engineers use the rule-of-thumb that anything under 60 volts
isn't likely to shock you. |
| Replacement lithium polymer
cells. |
Q: I am in search for a Walkman NW-S202F MP3
player replacement battery. Original is a polymer 116mAh 1-756-686-11
manufactured in 2006.
A: All you need is to find one that will fit into
the space of that battery. Here is our list, the ones with "add to cart" are
the ones we have in stock. You need to find whether the existing battery has a
protection circuit board mounted on the flange where the leads come out. It is
a small circuit board under the tape, but outside the battery pouch.
/li-pol.htm |
| Video-eye |
Yes, the man with the video-cam eye is using a
PowerStream battery in his eye socket. For more information about the eye, plus
borgeye video here is a link:
http://www.physorg.com/news/2011-08-eyeborg-vision-future-video.html
We did donate a few cells to that project. Too wild to resist.
Don't try this at home! |
| The patent office does another
disservice to the investing public |
I just got an e-mail invitation to invest in a
company based on patent number 7615876. Look it up, it is a fairly
straightforward perpetual motion machine which purports to use a gear box to
increase the torque and horsepower of a motor before it powers a generator,
which then powers the motor and a load.
So the lessons to carry away
from this is that the attorney, primary examiner, and possibly the inventor
should have known better than to let this slip through. And no investor can
depend on the patent office to evaluate the technical merit of anything. I
can't fault an inventor for trying to raise money on an patented idea that he
sincerely believes in, but I can fault the patent attorney, and especially the
patent office, which should have technical education enough to pull the plug on
this. Investigative reporter alert! |
| Excess power consumption in home
appliances? |
Q: Recently I've been a bit concerned about the
power consumption of my home appliances. I have a notebook power supply that
says: Input: 100-240V~ 1.7A 50-60Hz Output: 19V 3.42A Does this mean that it
consumes 187 watts if connected to 110 V and 374 watts if connected to 220
volts? Is it possible for it to consume twice the amount of watts if connected
to a 220 current? Thanks.
A: No, the current listed is the highest
possible, so that would be at 100 volts. As the voltage goes up the current
goes down to keep the watts the same. Also, note that the testing agencies (UL)
require that you put the highest possible surge current on the label, so you
cannot have any possible chance to calculate the efficiency or power
consumption of the device based on the label. best regards mark |
| At what voltage is a 9V battery
dead? |
I'm trying to find info on when a 9 volt
battery is sub par for use. I have a voltage meter, but the problem is that I
don't know the cut-off point where a battery loses effectiveness. For instance,
in a regular, non-rechargeable, Alkaline type battery, does a reading of 8.9
volts mean it's still has over 75% original capacity? What's the point where
battery should be replaced--at what voltage reading? I feel like I keep
replacing batteries installed in devices for back-up purposes should power
fail, when perhaps they would still do the job at a lower reading, but have no
idea what the readings really mean.
Thanks, Terri
Sorry Terri, there
is no good answer to this question. First of all, the voltage you measure is in
large part due to the chemistry voltage. The battery could have very little
charge left and still read high voltage when it is "open circuit." You have to
measure the battery under some load to get an idea of its state of charge. The
load that you would use would be drawing the same power as the gadget under
power. Next, it depends on the device the battery is powering. These transistor
radio batteries are usually used to power gadgets that don't take much power.
Also, some devices are designed to run over a wide range of voltages, so even
if the 9 volt battery is only putting out 5 volts they would still operate.
Lastly, 9 volt batteries consist of several cells in series. I have seen them
use 6 cells in series, and 5 cells in series. So a 9 volt battery could be
nominally 7.5 volts. Using 5 cells gives you less voltage, but the cells are
bigger, so they give more run time if the device it is running can use the
lower voltage.
The answer to your question can only be "When the device
can't operate, then the battery needs to be replaced." I know that this isn't
much help in your case, where the batteries are used for emergency purposes,
sorry again.
best regards
mark |
| Why can't you charge a lithium
ion battery when it is too cold? |
Mark, I've read (and heard you say) that a LiPo
battery should not be charged at low temps (e.g. -20C). Why is this? Is there a
safety reason for this or will it just degrade the capacity? --
Damien
The thermodynamics of the lithium-ion intercalation change below
about zero degrees C, and make it favorable to deposit lithium metal onto the
graphite. This seals the graphite and kills the battery, and lithium dendrites
have the chance to short the cells and cause a fire.
best regards,
mark |
| Time to recharge the battery
after starting the car. |
Hi,
There is a lot of discussion in my group
about how many miles you have to drive your car to recharge the battery after
starting. Can you shed some light?
Tonk
Dear Tonk,
I can do a
back-of-the-envelope estimate. Typical car batteries are rated for cold
cranking amps, and the one I use has 800 CCA. So being very conservative lets
assume that it takes 800 amps. I have three cars, 22 years old, 9 years old,
and 10 years old, they all start in less than 3 seconds, but to be conservative
let's assume 10 seconds. So 10 seconds times 800 amps is 8000 amp-seconds (8000
coulombs for you physicists) or 2.2 amp-hours.
Now let's look at the
charger. All of my cars have alternators greater than 60 amps, so 2.2AH/60A =
0.03 hours, or 2 minutes to recharge the battery.
More realistically,
lets say that it takes 300 amps to start the car for 3 seconds, that would take
0.25 AH, and the recharge time is 15 seconds.
Now the Karman Ghia that I
had in college usually took 2 minutes to start and had a 65 amp alternator. It
would have taken about 10 minutes to recharge.
best
regards
mark |
| Charging lithium ion in
parallel |
Hello Dr. Mark,
I'm glad I found your web
log. I have a question about charging Li-ion and Li-polymer cells in parallel.
I have searched for and found others who have opinions about this. Some say
absolutely don't do it because the cells won't charge equally. I know that is
an issue with cell in series, but does it apply to cells in parallel? Others
say it's safe because cells in parallel naturally balance because power will
flow from the stronger cell to the weaker cell. Can you shed some light in
this?
Thanks
Bruce
Dear Bruce,
The answer is that since
lithium ion chemistries are charged with voltage control they can be charged in
parallel with no problem. The same with lead acid batteries.
The nickel
based chemistries are charged with current control and they don't like to be
charged in parallel.
I have discussed this with many top battery
designers, and I have seen dozens of designs that charge lithium ion in
parallel with no problem, so I have high confidence in this answer. However, it
is rare for one battery to have enough voltage to charge another, nature just
doesn't work that way. What happens is that the battery with the lowest charge
state will take most of the current until their equivalent impedances are
equal. Since the lithium chemistries use a specific float charge voltage, if
you connect a fully charged battery with an uncharged battery very little
current will flow between the two. Then putting this combo on a charger the
fully charged battery will accept no current while the uncharged battery is
being filled.
best regards
mark |
| Mysterious Instrument |
A
correspondent sent me pictures of something she found in her parent's attic.
Any idea what it is?

Click on the picture for more views. |
| Switchmode or
Switching? |
Q; What do the words 'Switchmode mean? My
experience with switching power supplies is that they only work well if there
is some minimum load.
A: There are various topologies used in switchmode
aka switching power supplies. Some need to supply some output current in order
for the housekeeping circuitry to work, others have a separate bootstrap
circuit to make it operate. The former system is used in ATX computer power
supplies, the latter in almost everything else. best regards mark |
| AAA versus AA Capacity |
Q: Alkaline AA vs AAA amphours comparison
question for your battery guru: For Alkaline batteries what is the comparison
between AA and AAA batteries 1.5v in the amp hours output for say the 100ma
discharge rate (or any other rate for that matter). We want to know how many
AAA 's = 1 AA cell? what is the difference in amphours. thanks
A:
Depending on which ones you are comparing an AA has about 2 to 3 times an AAA.
/BET.htm
best regards,
mark |
| Mystery with battery
tender |
Q: I found your website while trying to solve a
problem. You seem very knowledgeable and I'm hoping that you wont mind helping
me (a newbie) solve a problem... I can't seem to charge a new 12V 18AH sealed
lead acid battery. I thought it would be a simple matter to connect my 750mah
Deltran Battery Tender however the voltage quickly runs up to 13.5v and the
Battery Tender discontinues output. Tried a second new battery tender, same
result. Tried a second sealed lead acid battery (12V 7AH), same result. Hmmpf.
So I tried a 1 Amp fixed output charging source. Voltage then quickly ran up to
15v after ten seconds so I disconnected it. If I put a moderate load on either
battery the voltage quickly drops to 8v. So they definitely are not charging.
What the heck am I doing wrong? Thanks for letting me lean on you.
-Mick
A: I can't see that you are doing anything wrong. I always assumed
that those battery tenders were like ours /SLA-12V07.htm
and would charge a battery as well
as tend it. As for your fixed voltage source, it won't hurt the batteries to be
charged at 15 volts until they are near full or full. Sorry, troubleshooting
from this distance is tough! Maybe one of our readers has some
insight.
best regards mark |
| Battery Capacity |
Q: When a battery’s “Rated
Capacity” is 170mAh, does that mean it is capable of delivering 170mA
continuously for one hour before the entire battery is discharged?
A: Yes,
but with limitations. The rating is measured over a specified time. Usually for
lead-acid batteries this time is 20 hours. For lithium-ion it is usually 5
hours. When discharging quickly you always loose some capacity.
This is a
very strong factor with lead acid, where if you discharge at a 1C rate you only
get 50% of the rated capacity. It is not a big factor in lithium ion, NiCad,
and NiMH batteries, unless you are discharging at very high rates (above 1C
to5C depending on the design of the battery) |
| Battery Capacity 2 |
Q: Is a battery’s “Rated
Capacity” linearly proportional? In other words, if a battery’s rated
capacity is 170mAh, could it be assumed that it can deliver 85mA for two hours,
42.5mA for four hours, etc.?
A: Yes, with the above limitation. mAH are
proportional to coulombs. 1 mAH = 3.6 coulombs. Other than the problem
discussed above it is linear. The slower the discharge the more linear it is.
If you discharge time is a period of months you will start to run into the
self-discharge rate of the battery, so you will again get less than the rated
amp-hours.
See our web page " Engineering Design Notes
on Battery Powered Devices " |
| Conservation of Energy |
Q: Hello: I have a question. I have a DC power
supply that is putting out 14 volts at 14 amps. And I have a motor that is
running at 14 volts but needs a higher amp rating. Can a DC-DC converter
increase the amp output? If not is there anyway to increase the amp
output?
A: Energy conservation means that "watts in" must be more than
or equal to "watts out." Watts is volts times amps, so if you increase the
amperage you have to decrease the voltage.
best regards
mark |
| Current limit of AC/DC
adapters |
Q: I have heard that AC adapters can output
TWICE the current as stated on the labels. Are there any that limit the
current?
A: The old-style transformers could do that and get hot, but
the voltage would drop, and eventually they would blow the internal fuse.
Switchmode power supplies can typically supply 120% with some loss in
voltage regulation (and they get hot), but start hiccoughing (turning on to see
if the problem has been solved, then turning back off again) when the current
goes too high.
If you want a current-regulated power supply, those are
harder to find, they are what battery chargers typically use.
I don't know
of any AC/AC power supplies that are current limited (other than the
fuse).
best regards
mark |
| Battery charging auxiliary
batteries |
Q: I run a computer repair business, and while
most of my work is done at the client’s site, sometimes I have to bring
their equipment back to my office. I’d like to set up a portable computer
lab in my van so I can do more work on-site, and have bought a couple 750AH
deep-cycle batteries to power things. I’d like to power the lab off these
batteries and not take any chances of running down the vehicle battery.
I’ve looked at your product list and wonder if your PST-DC-UPS-1212 is the
right product to keep the batteries charged while on the road.
A:
For such big batteries I would suggest the /battery-isolator.htm to charge while driving
and then isolate the deep-cycles from the starting battery. The PST-DC-UPS-1212
would take a long time to charge a 1500 AH battery! |
| Does turning a fluorescent light
on-and-off waste energy? |
Q: How long does a fluorescent light have to
stay "off" to pay for the energy required to start it up again?
A: This
is an urban myth from my childhood, and it isn't true now and I doubt that it
was ever true with the oldest iron ballasts. It does take energy to start a
bulb, but very little, insignificant compared with running the lamp for a few
seconds.
|
| Comments on the Stanford
Nanowire battery |
This link discusses a breakthrough at Stanford
University.
http://news-service.stanford.edu/news/2008/january9/nanowire-010908.html
This is interesting, but weird.
Since silicon isn't used in any
batteries at the present time, the article is somewhat misleading, evidently
the author missed some important facts. Carbon is typically used for the anode
material to store the lithium ions, and since silicon doesn't have a
graphite-like analog (Si crystallizes in the diamond structure)
Most
puzzling is the fact that the best lithium-metal batteries only have 2-3 times
the capacity of lithium ion batteries per unit weight or volume. Using
lithium-plated silicon nanowires you will have far less density than a solid
layer of lithium metal, so how can they have 10x the capacity? You can see from
the pictures that there is a lot of empty space in the nanowire tangle.
I can imagine that the cycle life could be 10x because there is no
wear-out mechanism, maybe the author got that confused. Or maybe the battery
has 10x the power output because so much surface area is in contact with the
electrolyte. But it can't have 10x the energy storage as stated in the article.
|
| Replacement batteries for
emergency jumper packs |
Q: Who in US sells replacement batteries for,
Jumper Battery packs used for emergency starting? Can't figure it out. Steve
A: These typically use standard-size sealed lead acid batteries, such
as those at /BB.htm. If you find one of the correct dimensions it will work.
best regards
mark |
| Why are lead acid batteries used
in standby applications? |
Q: Traditionally, lead acid batteries have been
used for telecom and off-grid electrical systems. If they can be damaged by
deep discharges, or by periods of partial charge, why are they used for these
applications? Could you use a NiCd battery in a UPS? Is there a better battery
chemistry for a renewable energy system? Thanks! -Tim
A: Their biggest
advantage is that they can be float charged, in other words kept at a certain
voltage without over charging. This makes them very convenient as batteries
that are rarely discharged, but are standing by for most of their lives, such
as telecom standby power.
NiCad can be used for UPS systems, but they can't
be float charged, so it takes a more sophisticated charge controller. Besides,
they are more expensive than the lead acid batteries.
For batteries
that are used in off-grid situations nickel-iron batteries are very nice. They
are virtually indestructible, can last for 50 years. They have a low voltage
and low energy density, and aren't as easy to find, so usually people use lead
acid instead.
best regards mark |
| Battery packs get hot |
Hi my name is Jerimiah.
I read a lot of
your tips online and have run into a situation I can’t figure out. I would
appreciate a little insight if possible. I have two battery packs that need to
be connected. They hold 4 AA batteries each. Unfortunately the manufacturing
company has gone out of business and I have no way of buying a new one. I have
connected both the packs exactly the same way they had them connected. When I
plug in the batteries they start to heat up. What do you think could be
happening? Thank you Jerimiah
You have some polarity backwards, either
one or more of the AA cells is backwards or the connection between the
batteries is backwards. If both wires from the one pack are connected to the
other pack then you should be in parallel, plus to plus and minus to minus. If
only one wire from one pack is connected to the other then you are in series
and you should connect plus to minus.
best regards mark |
| At what discharge rate are LiPo
batteries measured? |
Mark,
For LiPo cells, at what discharge
rate is the mA-hr rating based? I know for lead gel cells it is based at 20 hr
rate, with the A-hr reduced significantly at higher or faster discharge rates.
Are there published curves for given size LiPo cells at different discharge
rates? Mike
Dear Mike:
Typically it is the 5 hour discharge rate
that determines the capacity rating of a lithium polymer cell. Lithium cells
don't lose their capacity when discharged quickly as much as lead-acid do.
However, when the discharge rate gets to a few minutes they do lose some
capacity. Some data sheets will have the capacity versus discharge time.
Also, lithium polymer cells differ in their design. Some are designed
for rapid discharge for the RC industry and some are designed for slow
discharge for more sane applications. In the case of fast-discharge (10C to 20C
or 3 to 6 minutes) batteries the trend is to give the capacity at fast
discharge, since the 5 hour discharge rate capacity is irrelevant to the
users.
best regards mark |
| What is the truth about silicone
batteries? |
There has been a bit of flurry about a new lead
battery, the Silicone Power Battery. It is advertized as new, green, and cool.
To quote it uses a "silicate salt electrolyte that replaces the sulfuric
acid electrolyte of a normal lead acid battery. This produces a battery that is
environmentally friendly."
This excited the materials scientist in
me, since I know that 99% of silicon compounds are non-soluble, there must be
something cool going on. Besides, they say it is still a 2 V per cell chemistry
and is compatible with automobile charging systems.
Well, it turns out
that all this is just plain (mostly?) hype, or hyperbole whichever you prefer.
What they do is to gel the sulfuric acid with sodium silicates (a common way to
do it). So it is just a typical gel cell. They may be making a better gel cell,
I don't know yet, but it is nothing more or less than a lead acid gel
cell.
If you want to look up the patent application it is USA
application 20040175623. |
| Smaller rechargeable lithium ion
coin cells |
Many engineers have been
asking for smaller rechargeable lithium ion coin-cells. We are happy to
announce the new additions to our line of Li-ion LIR series cells, the LIR1220,
which is 12 mm in diameter and 2 mm thick, and the LIR1024, which is 10 mm in
diameter and 2.5 mm thick. |
| Why are non-rectifying contacts
important in CMOS? |
Dear Dr. Mark,
I know you are a former
JFET designer, but I have a question in general about metal contacts to
semiconductors.
I've read that depending on the n well process or the p
well process it is essential to diffuse p+ in a p-region or n+ in a n-region so
that an ohmic contact is achieved. Then, it is said that metal-semiconductor
contacts are rectifying, and that it is not "good" for the operation of CMOS.
Why is that? why do you need an ohmic contact so that current flows both ways
from metal to semiconductor?
What is so "bad" about rectifying contacts?
Thanks. Andres.
A: Dear Andres,
Rectifying junctions not
only prevent the current from flowing both ways, but also present a voltage
drop for forward biassed operation. This wastes power for simple circuits and
makes complicated circuits impossible to make. So the p+ or n+ implants or
diffusions are done to make a metallic-like silicon to contact to, which more
closely matches the fermi levels of the aluminum contacts.
Best regards
mark |
| 6 to 12 volt converters for
Volkswagens and Porsches |
We finally have released
a 6 to 12 volt converter that is 200 watts and fully isolated, so it can be
used in either positive or negative ground cars. This opens the 12 volt world
up to vintage cars that use 6 volt negative ground systems such as VWs and
Porsches.
Take a look at /dc6-12.htm |
| Using car batteries for deep
discharge |
Q: Car batteries are the cheapest because of
the high quantity manufactured. Why does everyone want to steer me toward an
expensive, special purpose, marine battery for deep discharge
applications?
A: Car batteries are mainly there for starting the car.
This takes a surprisingly high current, up to 400 amps for a big engine on a
cold day. The way they accomplish this is by having a lot of very thin plates
so there is a lot of surface area. This allows them to discharge quickly, but
they are easily damaged by deep discharging. Car batteries are normally rated
for only 5-10 deep discharges, sustaining damage with every one of
them.
Deep discharge batteries are designed to put out less peak current,
but they have thick plates that can be charged and discharged many times
without corroding or falling to pieces.
|
| Model helicopter charging
problems |
Hello, I'm Looking for advice on charging NiMH
batteries after having problems, I came across your web page - very informative
and helpful and answered some of my questions - many thanks for that.
I do
however still have some queries and I wonder if you could find the time to give
me further advice on this matter? My problem concerns charging batteries for my
model helicopter which uses seven AA NiMH batteries (8.4v).
When I
first purchased the model, the instructions said to charge the batteries for
three hours with the supplied charger. This I dutifully did (using a timer),
though the batteries became increasingly hotter after each charge, causing me
to reduce the charging time.
Within a very short time, the batteries
simply wouldn't power the helicopter and very little or no lift was generated.
I purchased new batteries, but the same thing is happening again. I can't help
but feel that the supplied charger is just not right for these batteries, it is
marked 12vdc and 300ma output and furthermore it says on the side of the
charger, (if that's what it is!) 12v adapter.
I would be happy to buy a
'proper' charger, but I can't find anything that will charge the seven cells
altogether. Any help or advice that you can give would be much appreciated,
Regards, Phil
Dear Phil, It looks like you just have a power supply
that is overcharging the batteries. I have used supplies like this to charge
NiMH, but you have to be careful to make sure that the current is less than
1/10 of the capacity when the battery is full. So for a 1000 mAH pack the
current should be less than 100 mA when the battery is full. You don't say what
the capacity of your batteries is, but I would be surprised if a 12 volt power
supply would work well for this. We have a smart charger at /NiMHWMm.htm
that will do it.
best regards
mark |
| "Paper" batteries from Rensselaer
Polytechnic Institute |
There has been a lot of press lately about the
Rensselaer Polytechnic Institute's battery made of cellulose and carbon
nanotubes I thought I would weigh in.
This is a great technical
achievement, however don't look for these in your cell phone soon, the cost of
carbon nanotubes is tremendous. Refined nanotubes sell for $300 per gram,
unrefined soot containing nanotubes $20 per gram. A 8.5 x 11 inch sheet would
take $36 worth of nanotubes. Due to the nature of nanotubes it is difficult to
imagine that they will ever be cheap enough for a consumer battery.
I
haven't been able to find the original paper to find out how many amp-hours per
square meter. I'll weigh in when I do. |
| Aircraft batteries are they 24
volts or 28 volts? |
Q: I am confused about the 28 volt airplane
systems. Do they use 28 volt batteries?
A: Cars and trucks and other
ground-based equipment traditionally use the "2 volt per cell" terminology. For
example a 6 cell battery is a 12 volt battery even though the open circuit
voltage is 12.9 volts and the charging voltage can be above 14.4 volts. This is
because the "average" voltage as a lead acid battery is discharging is about 2
volts per cell.
Aircraft have used a different standard. They typically
quote the alternator voltage rather than the battery voltage. This make sense
because (even for ground equipment) most of the time the system is running at
the alternator voltage, the battery itself never gets discharged very much. The
alternator voltage is nominally 13.8 volts for a "6 cell" system and 27.8 volts
for a "12 cell" volt system. This is typically rounded to 14V and
28V.
Therefore a 28 volt aircraft electrical bus will have a 24 volt
lead acid battery in it.
So the answer is "No, 28 volt aircraft use 24
volt batteries." |
| Parallel and Series in pallet jack
batteries |
Q: Hello, I have a quick question regarding
pallet jack batteries. We use Hyster, 24 volt systems. When some of these came
in, they contained 4, six volt batteries, wired in series. Some have 4, twelve
volt batteries, wired parallel.
How do you determine the amp - hour
capacity for the correct charger. Wired parallel, do you add the capacity of
all four batteries or is it divided by two? I have asked this question to two
people who work in the battery industry and actually got two different
answers.
Dear David, When in series the voltages add, but the amp
hours stay the same. When in parallel the voltages stay the same but the amp
hours add.
If you have a 24 volt system with four 12 volt batteries
then you have two in parallel and two in series, so the pack would have twice
the voltage and twice the AH of a single 12V battery.
best regards mark
|
| What is happening to Lead Acid
Battery Prices? |
Q: Why are lead acid battery prices going
ballistic?
A: You are right, prices for lead acid batteries are going up
faster than oil. There are a couple of issues that have caused it. The first
was that China has taken the VAT tax exemption for exports of lead acid
batteries off because lead acid batteries are no longer a preferred
export.
Secondly, the price of lead has been skyrocketing. Here is a
chart of the price of lead from August 2000 to August 2007. You can see that
the prices were fairly stable for several years, then took a 100% increase for
a year or so. Now lead is trading at 7 times what it did 4 years ago.
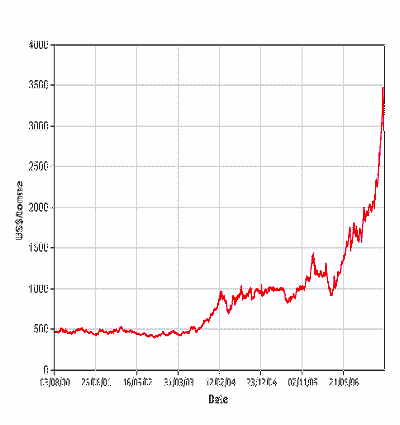
The next chart is over one year between August 2006
and August 2007.
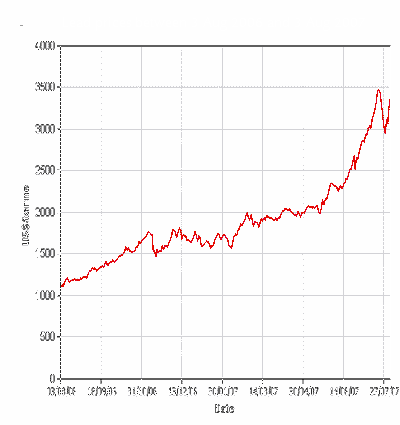
This shows that over the last year the price has gone up about
300%, this has really played havoc in those industries that use lead,
particularly for lead acid batteries.
We estimate that for every $100
per metric ton increase you can expect a 4% change in the cost of a lead acid
battery. This alone would say that the price should have increased 80% over the
last year.
This, of course, plays havoc with those of us in that
industry. Some factories have lead stockpiles that allow them to smooth out the
price increases, but this means that even if lead plateaus in price they will
eventually run out and need to raise their prices. Unless the price of lead
drops soon there will continue to be increases in prices.
I can also
vouch for the fact that no-one stockpiles raw materials these days.
best
regards
mark |
| 24 volt batteries |
Q: Hello, I'm just curious why 24V batteries
are not more popular or even used, or available. Thanks, Mark H.
A: Dear
Mark,
24 volt batteries are very popular, they are the standard for
wheelchairs, used on heavy earth moving equipment, the standard for Semi's in
Europe, many electrical bicycles, yachts etc.
But they are never, ever
sold as 24 volt. They are sold as 12 volt batteries, then connected together in
series by the user.
best regards
mark |
| Parallel and Series lead acid
batteries |
Q: Dear Sir or Ma'am, I am considering
purchasing one of your sealed lead acid battery chargers, but I have some
questions that I'd like to ask to make sure that I am buying the right charger.
The batteries that I am trying to charge are 6V 12AH sealed lead acid
batteries. Is is possible and/or safe to connect two of these 6V batteries in
series (essentially creating one 12V 12AH battery) with either your 12V 6A car
battery charger or your 12V 35A car battery charger?
Assuming that is
both possible and safe, would it also be safe to charge two of those composite
12V 12AH batteries with one of those car battery chargers by connecting the two
12V 12AH batteries to the charger in parallel?
Is it safe to place a
load (12V, up to 10 Amp load) on the batteries while they are being charged
with one of those car battery chargers? Is there a formula to determine how
long it will take for a sealed lead acid battery to charge based on the current
output of the charger and the amp-hour rating of the battery? Thank you very
much for your assistance. Sincerely, Daniel
A: Dear Daniel, Thanks for
your letter. Yes, it is appropriate to charge two 6 volt batteries in series.
It would be best if they were at the same state of charge (or discharge) before
being connected in series and either discharged or charged. You can also charge
two sets in parallel. Again, if they aren't approximately at the same state of
charge it might confuse a smart charger into slowing down the charge
prematurely.
A 10 amp load might confuse a smart charger. Adding a load
in parallel is done all the time, but for best results you need a charger that
is designed for continuous duty, most car chargers are not, they will overheat
if left on a 10 amp charge continuously. Also, smart chargers might time out,
or cycle on and off under these conditions.
These chargers were designed
for this kind of application: /charger-low-noise-high-power.htm
Amp-hours are just that, amps times hours. So a 7 AH battery should
take 7 hours to charge if 1 amp is applied to it. However, it typically takes
more time to get the last 5 or 10% into the battery because the voltage of the
battery goes up and unless the charger has an accurate way of determining the
end-of-charge it isn't safe for the battery to keep up the constant current
until it is full. So it takes a little longer than you would expect. Also there
are some charge inefficiencies that make the amp-hour law a little inaccurate.
best regards
mark |
| Running LCD TVs off RV Power |
A: Hello, I have a 15" flat panel TV that I
usually plug into an AC wall outlet in my home. Instead, I want to run this TV
from a 12v battery in my RV when I am camping in an area where AC power is not
available. I know that I can purchase a DC>AC inverter that will do this
but, is it really necessary since the power adapter that came with the TV shows
that the set runs on 12v DC 4.16 amp. So, if my TV ultimately requires 12v DC
4.16 amp input, why wouldn't I simply run it directly from the 12 volt RV
battery instead of converting DC>AC and then back to DC? Is there a simple
solution to this problem? Thanks, Frank
A: If the TV can handle the
range between 10 and 15 volts you can run it directly off the RV battery. If
not you need to stabilize it to 12 volts. /dc-buck-boost.htm has the
PST-DC/2812-8 which would do the stabilizing. |
| Capacity at end-of-rated-life for a
battery |
Hi, I am writing a report on batteries, and I
had a question regarding charging cycles. I see that the battery comparison
chart specifies how deeply the battery is discharged, and how many times that
battery can be charged. But what exactly does it mean that the battery can no
longer be charged?
Does that mean the battery no longer charges beyond
a certain capacity? For example, if a battery has a nominal 1500mA-hr and is
said to be able to go through 700 cycles, then after the 700 cycles what is the
new amp-hour rating? Please let me know what you know about this topic, or if
there's any good literature. Thanks a lot > > ~James
Dear
James,
The industry standard is that the battery is at its end of life when
the recoverable capacity reaches 80%. So a 10 AH battery would only have 8 AH
when considered to be at its end of life. Of course the batteries can still be
used way beyond this 80% mark.
I used to have a cell phone that would
only last for 2 calls per day, but that was enough to get a call from my wife
to remind me to come home from the lab. :) |
| Voltage of a generator |
Hi, I found your website to be very helpful.
Is there any way I could convince you to offer the same level of
explanation to this question: when I spin a 12V DC motor backwards to generate
electricity, how is it possible to generate voltage greater than 12V?
Thanks, Dave
A 12 volt motor isn't really a "12 volt" motor. It
is just rated to rotate at a certain speed when 12 volts is applied.
If
more voltage is applied it will spin faster, and if less is applied it will
spin slower.
The voltage generated when used backwards is proportional
to how many wire loops are cutting how many magnetic flux lines per second.
Best regards mark |
| Are silver oxide cells
"alkalines?" |
Hi. I was just looking at some batteries on
your website. I'm writing because I have a basic question as to how to
categorize silver oxide button cell batteries, and you seem very knowledgeable.
I would really appreciate any clarification you can offer.
My question
is this: I understand that a silver-oxide button cell battery (like the SR1120W
and other SR batteries) has a silver oxide cathode and uses an alkaline
electrolyte. Does the use of the alkaline electrolyte (potassium hydroxide or
sodium) make these batteries "alkaline batteries?" Or are they not "alkaline
batteries" because they have a silver oxide cathode? Also, what does the "W" at
the end of a battery designation (like SR1120W) mean, as opposed to say an "X"?
Thank you so much for any help you can give me! -
-- Andrew
Hi
Andrew, A battery engineer would say "this is an alkaline battery" because it
uses a KOH electrolyte.
But a consumer thinks that a manganese
dioxide-zinc battery with a KOH electrolyte is what is meant by "alkaline" due
to the perpetual dumbing down of the populace by marketing types.
This
opens up a confusion, because there are zinc alkaline button cells with about
the same voltage and size as the silver variety. But you can pretty much count
on the fact that when button cells are advertised as "alkaline" they are the
lower capacity manganese-zinc alkaline and not the silver-zinc alkaline. I know
about the prefix, S means silver-oxide-zinc and R means round, but I don't know
what the suffixes mean.
best regards mark |
| How low can a NiCad or NiMH
go? |
Hi,
Can you discharge NiMH/NiCad cells to
<1.0 volts and get away with it? Say down to .3V-.4V..
Scott
Dear
Scott,
A single cell can be discharged to zero volts without damaging
it. However in a pack there is always the danger of reverse charging one of the
weaker cells when the pack voltage gets too low. This causes damage to the
cell, which makes it liable to be reverse charged even deeper the next cycle,
etc.
best regards mark |
| Corrosion from NiMH |
Dear Dr Mark,
A
considerable number of circuit boards fitted with NI-MH batteries (not
purchased from PowerStream) are being returned with severe corrosion of the
copper tracks and components within a 2” radius of the battery, (gold
plated pins have a blue powdery corrosion).
The corrosion also softens
the plastic PCB and causes capacitive leakage/breakdown between the copper
tracks. The batteries are 3.6V 150mAh with an average charge voltage of 4.1V
and retain their full charge even when badly corroded. Most of the boards come
from petrol pumps but the problem also exists on computer boards and POS
circuits. The corrosion effect continues after cleaning the board and replacing
the battery.
Q ...Assuming the corrosion is caused by gas coming from
the battery can you tell me what chemical is causing the damage and recommend a
treatment I can apply to the circuit board to remove/stop the corrosion process
prior to replacing the battery?
Thanks Tom
PS .. Brilliant
site
A: The electrolyte is primarily potassium hydroxide solution KOH,
otherwise know as potash. It is corrosive, but quickly reacts with atmospheric
CO2 to form potassium carbonate which isn't corrosive, and neither are
poisonous.
The batteries will only vent if they are being over charged,
which builds up gas pressure in the cell. If this pressure gets too high it is
released by the safety vent in the cell to prevent explosions. Such pressure
relief does not kill the battery, it only reduces the capacity slightly.
With the gas some of the liquid comes out, which contains the KOH. I
wouldn't have expected the corrosion to continue after cleaning the board. KOH
is extremely soluble, so deionized water should take it off.
Potassium
carbonate is harder to wash off, it isn't as soluble, but it isn't corrosive
either. |
| Charging the starting battery with a "house"
battery |
Q: Dear Dr. Lund:
I was
wondering if the PST-BC1212-15 DCDC Charger would be
appropriate for the following use: If I am carrying a house battery on board my
van being charged with a battery to battery charger from the start battery, and
then the start battery is inadvertently run down (say I leave the headlights
on)… can I then reverse the wiring to use the battery to battery charger
to charge the start battery from the reserve in the house battery? I would use
sufficient gauge cable to prevent more than 2-3% voltage drop. I am hesitant to
connect the house battery directly to the start battery… is this scenario
a feasible solution? Am I being too careful in not wanting to make a
“jumper” type connection, instead using the battery to battery
charger?
A: Yes, this would work, and not a bad idea at all. Usually the
house battery isn't designed to put out enough current to start the van, it is
designed instead to deep cycle. If it is large enough to even give a partial
charge to the starting battery it could get the van started.
You need more
voltage to charge a battery than another battery would supply, which is why the
PST-BC1212-15 would be needed to do the charging. It has a DC/DC converter to
boost the voltage.
And our charger isolates the house battery from the
van so that it won't run down if you leave the headlights on. |
| New Product, 24V to 48V DC/DC
converter |
Announcement
We just released the new
DC/DC converter for running 48 volt equipment in 24 volt vehicles. This is
useful for wireless routers and bridges on low voltage systems. This
compliments the 12V to 48V converter we have sold for a couple of years.
|
| Circular Mils |
Q :
For transformers the current carrying capability of wire is often listed in
"circular mils" What is a circular mil, and how do I relate it to the real
world? Thanks a lot
A: The unit "circular mils" is a strange unit. It is
a measure of area--the area of a circle 0.001 inches in diameter, which turns
out to be 7.85 x 10^-7 square inches, or 0.000507 square mm. So if the
criterion is 500 circular mils per amp and you need 7 amperes you would
multiply 500 * 7 = 3500 circular mils, multiply by 0.000507 to get 1.771 square
mm, or a wire with diameter 1.5 mm, or about 14 AWG. |
Free power from the Phone
Company!
Are shallow discharges equivalent to
deep discharges?
Pee-powered battery, hype or hoax?
How much voltage is required to charge a lead acid
battery?
|
Can you really steal power from the
telephone company?
There is an
infamous company (that I won't link to for the world) that sells appliances
that run off of a telephone outlet (even vacuum cleaners). Is this real? Can
you really “stick it to the man” by tapping power off of the greedy,
faceless phone company?
Let’s do
the numbers:
It turns out
that there really is 48 volts on your telephone outlet. The current is limited
to at most about 50 mA, and most phones run on 20-30 mA. If you draw more than
50 mA on a the phone company will probably put you down for a short and cut off
your phone service until the short is fixed. When you start to draw any current
at all from the phone line the 48 volts sags considerably, but we will use 48
volts for our calculations just to be conservative.
(Don't forget
that the ring tone is 90 volts, 20 Hz, and it can go up to 120 volts, 20 Hz, so
don't get shocked while messing around.)
So, say you
wanted to get free power. The 20 mA at 48 volts is about one watt. Here in Utah
we pay about $.07 per kilowatt-hour. So by tapping power from the phone line
you could save seven cents in 1000 hours, or 41 days. Now you are
sticking-it-to-the-man to the tune of 61 cents per year.
The company
that makes these so-called products sells a little voltage converter for $70.00
to get you started in the free-energy business. You can pay for it with your
savings, breaking even in just 114 years. After that it is all 100% pure
savings.
Now, just who
is that “man” that is getting the stick stuck to?
Are shallow discharges equivalent to deep discharges?
A recent blog posted on
Zdnet
implies that you can wear out your laptop battery by leaving your battery in
the laptop and letting it recharge every time you plug it in. This is
misleading. To paraphrase the poster, he said that the battery is only good for
300 charge-discharge cycles, so leaving your battery installed every time you
boot up from AC power will wear it out after only 300 boot-ups.
The
truth is that a lithium-ion battery that is rated for 300 full discharge cycles
is also rated for thousands of shallow discharge cycles. A rule of thumb in
battery design is that the life of the battery is related to the total number
of electrons drawn from it, therefore 300 full discharge cycles is equivalent
to 600 half discharge cycles, or 3000 1/10 discharge cycles.
As with all
rules of thumb the relationship is only approximate, in this case the cycle
life actually gets better than you would expect for shallower
discharges.
You can verify this from almost any cell data sheet, but
I'll just list one other witness to this fact, this one from Motorola
(unfortunately the link to the Motorola article is no longer active) :
The relationship between DOD [depth of discharge] and cycle
life is logarithmic. In other words, the number of cycles yielded by a battery
goes up exponentially the lower the DOD. Research studies have shown that the
typical cellular phone user depletes their battery about 25 to 30 percent
before recharging. Testing has shown that at this low level of DOD a
lithium-ion battery can expect between 5 and 6 times the cycle numbers of a
battery discharged to the one hundred percent DOD level continuously.
A recent science news story that I really need to comment
on:
The first urine-powered paper battery has been created
by physicists in Singapore. The credit-card sized unit could be a useful power
source for cheap healthcare test kits for diseases like diabetes, and could
even be used in emergency situations to power a cellphone, they say. . .
The same story was repeated all over, look it up on Google if you
want to read it. I am reluctant to dignify it with a link.
The sad thing
about this is that the battery does not take its power from urine, it is just a
dry-charged battery that needs some water to activate it. I have some 30+ year
old army surplus batteries somewhere squirreled away in my "neat-science-stuff"
collection that work the same way, using the exact same chemistry. The battery
is completely inert until you add water to it, and it isn't too picky about
what is also dissolved in that water, or where (or who) it came from.
Luckily the article goes on to explain the chemistry to us technical
types:
The battery is made of a layer of filter paper steeped in
copper chloride, sandwiched between strips of magnesium and copper. This
"sandwich" is then laminated in plastic to hold the whole package together.
The resulting battery is just 1 millimetre thick and 60 by 30 mm across
– slightly smaller than a credit card. To activate the battery, a drop of
urine is added and soaks through the sandwiched filter paper. The chemicals
dissolve and react to produce electricity. The magnesium layer acts as the
anode, losing its electrons. And the copper chloride acts as the cathode,
mopping up the electrons.
So it is just a magnesium/copper chloride
battery just like my WWII army surplus one. (Not rechargeable, though the
article from New Scientist implies that another drop will reactivate the
battery). The claim of "first" mentioned above is hard to understand.
To
learn more about reserve batteries, go to /BatteryFAQ.html#MgCu
Q: I am trying to charge batteries with "alternative energy"
which does not give a steady voltage. How much voltage is required to charge a
lead acid battery?
A: The minimum voltage necessary to charge a
lead acid battery is 2.15 volts per cell, or 12.9 volts for a 12 volt battery.
This is what is needed to get the battery chemistry moving in the right
direction. Anything less than this will not do anything.
At this
voltage the battery won't accept very much current, or in other words it won't
charge very fast. There is a band of voltage between 2.15 and the 2.39 volts
gassing voltage when the water starts to disassociate (12.9 and 14.4 volts for
a 12 volt battery) which is the charging range. The higher the voltage the more
current you can stuff in the battery.
Some schemes go way above 14.4
volts to get a rapid charge, but drop their voltage when the battery nears full
charge.
Depending on the voltage range of your energy source, you may
need a DC converter to efficiently use the power generated, and possibly a full
buck-boost converter.
Don't stop, Click Here for the Dr. Mark Blog Archives!
|
