
| April 26, 2024 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Engineering Guidelines for Designing with Batteries |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Battery design studio
Mechanical Considerations1. Batteries are not made to precision tolerances. Allow ±0.5 mm tolerance for both diameter and length for cylindrical batteries, and as much as ±5 mm tolerance for lead acid and sealed lead acid parallelepipeds. 2. Batteries also expand and contract during charge and discharge. Potting a battery is not a good idea, unless there is some provision made for this dimension change, such as a thin layer of plastic foam, or flexible potting material. 3. Over the course of life Nickel Cadmium, Nickel Metal Hydride, and Lead Acid batteries release hydrogen, and sometimes oxygen. Take this into account if you are designing a closed system, such as waterproof lights, weatherproof installations, etc. Some method of releasing or absorbing the hydrogen, flooding with air or inert gas should be used. In closed cabinets some provision for ventilation is necessary to prevent hydrogen gas from accumulating. 5. When using battery packs be careful not to inadvertently short the cells. A pack of cells wired in series will become shorted if the cases of adjacent batteries touch, since the outer case is a terminal. This can happen if the cells are shrink wrapped, film wrapped or painted and the batteries rub against each other. Brittle shrink wrap is known to shred under stress, and at high temperatures, leaving the bare cell walls to touch. 6. When using or designing battery holders make sure there is adequate provision for short cells, long cells, or wide cells. Keep sharp clip edges from touching the cell where they could cut the film or paint, causing a short between cells held by the same clip. 7. Position the cells away from heat sources if possible. Heat will cause an increase in self-discharge, and will also decrease the life of the battery. The old rule-of-thumb of twice the self discharge every 10°C increase in temperature still holds. 8. Don't measure the length of a live cell with metal calipers! Be careful--batteries are different than other components, since they store energy. They can short from keys, from metal tools, or coins, so be careful of putting them in your purse, tool box, or pocket. 9. When making fixtures to hold removable cylindrical batteries use contact forces of 1-2 pounds, depending on the weight of the battery and the roughness of handling expected. 10. There is a helpful ANSI standard C18 which gives a lot of guidance for battery power devices. 11. Double-sided sticky tape. Many major manufacturers are now holding lithium polymer and lithium prismatic cells down using double sticky tape. This is an extremely secure way to hold these down, they cannot jiggle loose, and even rework takes a lot of prying. An example of the tape that we use is the 3M 9472LE series tape, 240 micron base with 130 micron adhesive layers. Electronic Considerations1. If there are alternate ways to power your device, make sure to isolate the battery from the alternate sources of power to prevent inadvertent charging. In the case of high voltage batteries isolate the battery from terminals of alternate power sources. This is particularly important with non-rechargeable cells, which could vent, leak, or even explode if a small amount of leakage current from the main power source gets into the battery. For the lithium metal non-rechargeable batteries one or two diodes in series to prevent charging is often mandated. 2. Some cells, such as CMOS maintenance lithium batteries are designed for a very low rate of discharge. Some meters will load them down with too much current, and so will give incorrect readings. Make sure you are using a high impedance meter. 3. Open Cell Voltage (voltage measured with a high impedance meter) is a function of the cell chemistry and internal leakage. Even dead cells can register the same open circuit voltage (OCV) as good cells. The correct way to test a battery is its to measure its voltage under load. 4. Be careful to match the cells in a battery pack. When a battery pack is near zero volts under load the weaker cells will go into reversal, and suffer damage and perhaps vent. 5. When using rechargeable cells, don't over-discharge below the rated discharge voltage. For NiCad and NiMH this is about 1 volt per cell. For lead acid, it is about 1.75 volts per cell (10.5 volts for a 12 volt battery). Rechargeable lithium batteries must have a provision to prevent them from discharging below about 2.5 volts per cell. This is to prevent the copper electrode from corroding and killing the cell. 6. Take care when designing a battery holder which is intended to connect the cells in parallel. A cell that is accidentally inserted backwards will short out the pack. In addition, customers typically expect the batteries to alternate orientation. 7. How much capacity do I need? Click here for a tutorial. 8. There should be some provision for removing the battery from the circuit when the voltage gets too low. This will prevent leakage and corrosion from non-rechargeable primary batteries, and extend the service life of rechargeable batteries greatly. Designing with Alkaline and Carbon-Zinc BatteriesWhereas rechargeable batteries tend to be rated at a nominal voltage, primary batteries are most often rated at their peak voltage. In the case of the manganese dioxide-zinc chemistries (alkaline, carbon-zinc, Leclanché) the rated voltage is 1.5 V, but this is only the peak voltage. Since there is no pronounced hook at the end of charge, these cells are serviceable down to 0.6 volts. so (1.5 + 0.6)/2 = 1.05 volts might be used as the nominal voltage. Many engineers have made the mistake of designing their equipment for an alkaline battery voltage range of 1.5-1.2 volts, meaning the cell becomes unusable when 70% of the chemical energy is left in the cell. Don't be among them! Reverse polarity protectionIf the customer puts his Alkalines in backwards will your circuit survive? The most obvious choice is to include a diode in series with the battery to prevent reverse polarity.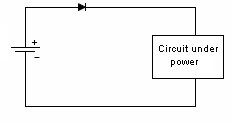 But this adds a voltage drop across the doide, wasting battery capacity and voltage. Another method is to use a MOSFET, which has very little channel resistance. The gate is operated by the battery, either in correct or incorrect orientation, turning the transistor off when the battery is backwards. Alternately, a p-channel MOSFET can operate on the other leg, with the gate connected to the negative rail. The FET is chosen to allow the needed current to flow without exceeding the recommended current, and may need to have a heat sink. 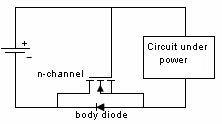
Capacity of alkaline batteriesIt is almost impossible to get the manufacturers of alkaline cells to give a capacity in amp hours, but here are some approximate numbers. The first column is a generic list, rated for 60+ hour discharge rates. For 2-4 hour discharge rates multiply the capacity by 0.4, for 5-10 hour discharge rates multiply by 0.6, for 15-20 hour discharge rates multiply by 0.8, and for 50 hour discharge rates multiply by 0.92. The the other columns are famous brand with the manufacturer's trademark slightly obscured. Charts measuring the different brands are linked to below.
* For Discharge Curves for 9 volt batteries click here ** For Discharge curves for AA alkaline and NiMH cells click here Designing with Zinc/Air batteriesThe zinc air battery is a wonderful thing if you have the right application. Since you don't have to carry the oxygen source with you, it has very high energy density. For more information about these cells see the battery chemistry FAQ listed at the top of the page. The voltage versus discharge of these cells is amazingly flat, at about 1.2 volts. Since the electrolyte is exposed to the air it will loose water. At low relative humidities (such in Utah, where we live) the voltage will start to sag with 40% of the capacity remaining, but at 75% relative humidity it stays up above 1.2 volts for 95% of the capacity. The cell can said to be exhausted at 1 volt, since at this point the voltage is dropping like a slam dunk. Product Considerations1. It is better to over-design the battery run time than to under-design. Under-design might save some costs, but the customer will perceive that you have a poor product if the battery life is lower than he expects. This is a lesson to be learned from PDAs. They use a lot of power, but they must be small, so they use disposable alkalines, which give twice the run time of rechargeable batteries. 2. Remember that most people will remove the batteries from a device before noting their orientation. Clear instructions on the position, orientation, and order of new battery installation are expected. 3. You have a choice between using off-the-shelf or custom, common or hard-to-get batteries and packs. Using common, locally available batteries saves money in development cost, development time, and logistics. But sometimes a common battery size won't work. Another consideration in some industries is theft. An equipment manufacturer that decides to use a commonly available camcorder battery for his equipment might find that his industrial customer is spending a lot of money to replace stolen batteries. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
| |||||||||