
| February 7, 2023 | |||||||
Vapor Pressures of the Chemical Elements |
|
||||||
|
Custom design and manufacture of state-of-the-art battery chargers, DC/DC Converters, and power supplies I occasionally need to evaporate some metal or other, or want to know if my ampule will explode at 1100 degrees C, so as a ready reference I have included these on our web page. Note: for a higher resolution version click on the graph. Note that the "boiling point" measurement is at one standard atmosphere. 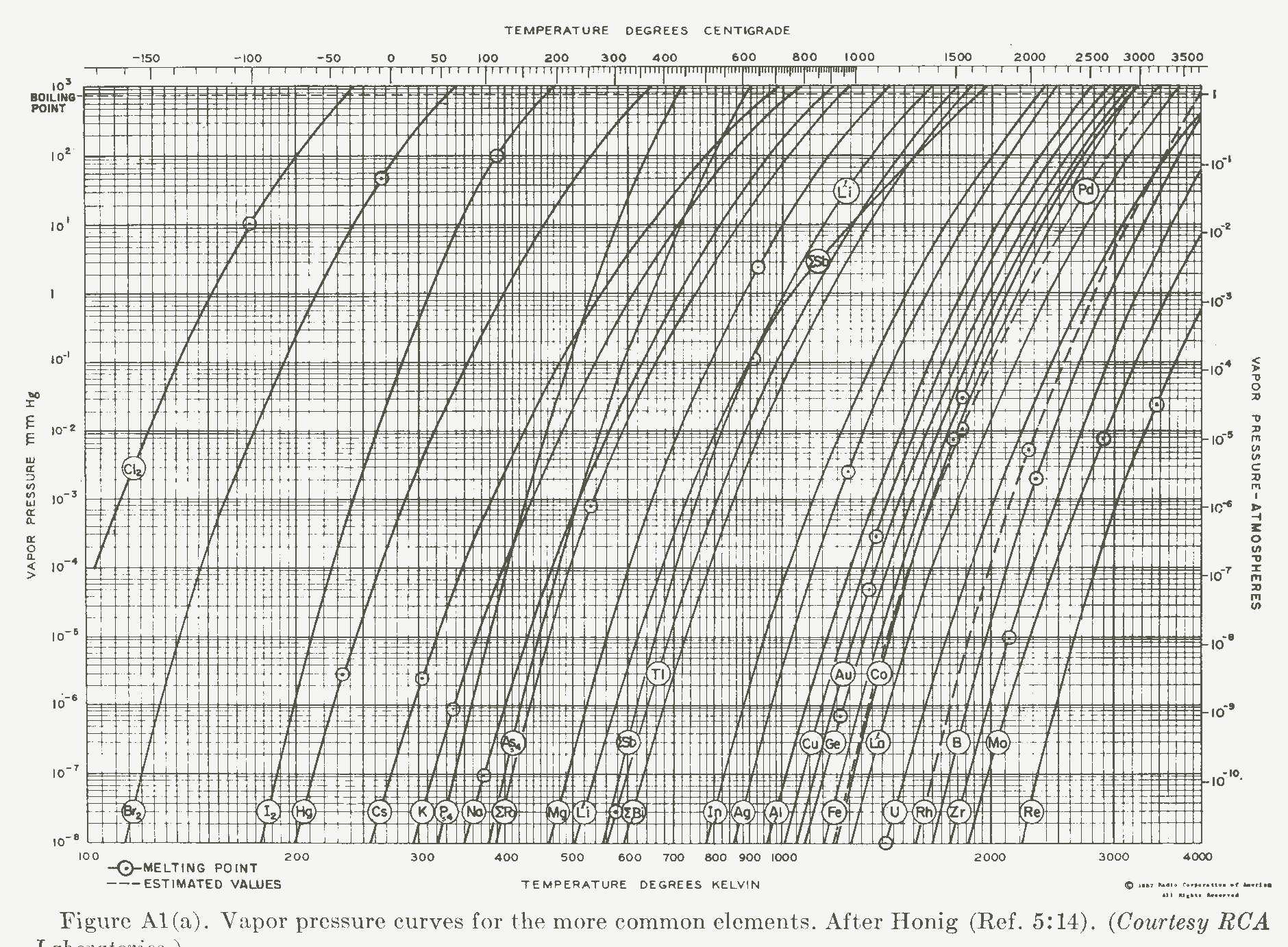 Vapor pressures of chlorine, bromine, iodine, mercury, cesium, potassium, phosphorus, sodium, arsenic, potassium, magnesium, lithium, antimony, titanium, bismuth, indium, silver, aluminum, copper, gold, germanium, iron, cobalt, lanthanum, uranium, rhodium, boron, zirconium, palladium, lithium, molybdenum, and rhenium. Note: for a high-resolution version click on the graph: 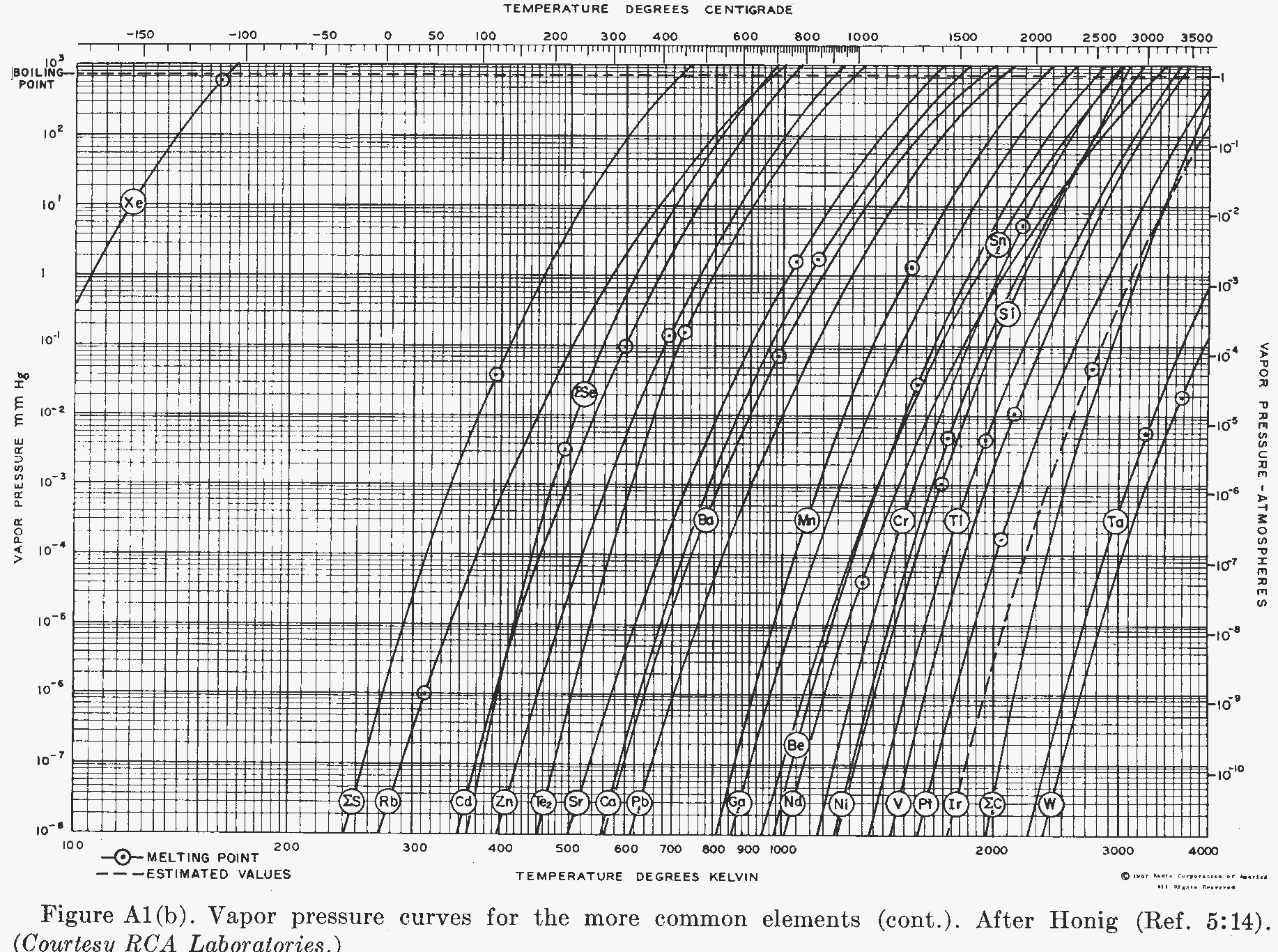 Vapor pressure of xenon, aluminum, tungsten, iron, nickel, copper, gallium, lead, calcium, strontium, zinc, cadmium, rubidium, sulfur, carbon, iridium, platinum, vanadium, neodymium, beryllium, tantalum, thallium, silicon, chromium, tin, manganese, barium, and selenium. How do you measure the vapor pressure of refractory elements, such as Tungsten? There is an interesting paper that describes the measurement and the care that you need to take in doing it: "The pressure and heat sublimation of tungsten" by Szwarc, PLante, and Diamond at what was then the National Bureau of Standards. https://nvlpubs.nist.gov/nistpubs/jres/69A/jresv69An5p417_A1b.pdf |
|||||||
|
|
|
| |||||||||