|
Batteries come in a lot of different varieties, and many
years of work at universities, government labs, industrial workshops and
inventor's basements have been put into investigating thousands of different
chemistries. The most common are carbon-zinc, alkaline, lead acid, nickel metal
hydride, nickel cadmium, lithium iron phosphate and lithium ion. But there are
many other commercially available battery chemistries, each with their own
advantages and disadvantages. Below we discuss different of the battery designs
currently used, some of the chemistry involved, and advantages and
disadvantages of each design. We have also included some useful definitions and
a list of parameters to guide you in matching your battery requirements to a
specific battery design.
- More Technical
Resources on the PowerStream Web Site
Table of Contents
- Anode: The electrode where oxidation (loss of
electrons) takes place. The anode of a battery is the negative electrode when
discharging, and when charging it is connected to the negative electrode of the
charger. So when the anode is charging it technically receives electrons,
making it the cathode, but no-one ever makes this distinction due to the
confusion it can cause.
- Amps: Also known as Amperes. This is the
rate at which electrons flow in a wire. The units are coulombs per second, or
since an electron has a charge of 1.602 x 10-19 coulombs, an amp is
6.24 x 10+18 electrons per second. Think of marbles rolling through
a tube. If 6.24 x 10+18 pass by in 1 second you would have an amp of
marbles.
- Amp hours: Also known as ampere hours.
This a measure of the amount of charge stored or used. For example if you had
an amp of marbles flowing out of your tube into a bucket for an hour, you would
have one amp-hour of marbles in the bucket ( 6.24 x 10+18 times 3600
seconds = 2.2 x 10+22 marbles. A 1 amp hour battery contains enough
charge to supply 1 amp for 1 hour, if you discharge at a constant rate of
current. But note that usually if you discharge faster than the rate at which
the the amp hours were specified you will get fewer amp-hours out, see the
definition of Peukart Effect below.
- You may notice that amp-hours and coulombs measure
the same quantity-charge. One amp-hour is 3600 coulombs, but amp hours are
easier to use in battery design. So remember, amps are flow ( "this motor
requires 2 amps to run at 1800 rpm.") Amp hours measure capacity, quantity, or
amount of charge ("this 100 amp-hour battery will supply 2 amps for 50 hours
before needingrecharge." Amp-hours are amps times hours, not amps
divided by hours.
So Amp-Hours, (AH), or milliamp-Hours (mAH) is a
measure of the size of the battery a 10 mAH battery has half the capacity of a
20 mAH battery, even though they may be in the same physical package.
- Batteries: Two or more electrochemical cells,
electrically interconnected, each of which contains two electrodes and an
electrolyte. The redox (oxidation-reduction) reactions that occur at these
electrodes convert electrochemical energy into electrical energy. In everyday
usage, 'battery' is also used to refer to a single cell, but technically it is
a combination of two or more.
- C:C represents the capacity of a battery divided
by 1 hour, its units are amps. It represents a 1 hour discharge rate using the
nominal capacity of the battery. So a discharge rate of 10C for a 5AH battery
would be 50 amps. A discharge rate of C/10 would be 0.5 amps. The concept of
"C" is also used for charge currents, since both charge and discharge
properties are proportional to the capacity of the battery, so a 5C charge rate
for a 5 AH battery would be 25 amps.
- Capacity: The total quantity of electricity or
total ampere-hours available from a fully charged cell or battery.
- Cathode: The electrode where reduction (gain of
electrons) takes place. The same confusion applies when charging as the anode,
but the cathode remains the positive side of the battery to avoid
confusion.
- Charge: The conversion of electrical energy,
provided in the form of current from an external source, into chemical energy
stored at the electrodes of a cell or battery.
- Discharge: The conversion of the chemical energy
of a cell into electrical energy, which can then be used to supply power to a
system.
- Discharge curve: A plot of cell voltage over
time into the discharge, at a constant temperature and constant current
discharge rate.
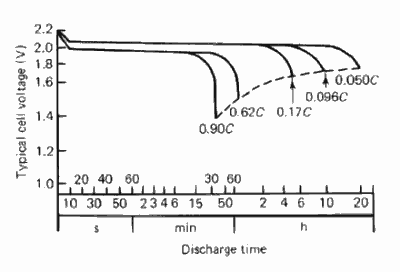
Each curve in this graph represents cell
performance at a different discharge rate. The farther right the curve ends,
the lower the discharge rate (Crompton 31.4).
- Dry cell: A
Leclanché cell, so called because of its non-fluid electrolyte (to
prevent spillage). This is achieved by adding an inert metal oxide so that the
electrolyte forms a gel or paste.
- Efficiency: For a secondary cell, the ratio of
the output on discharge to the input required to restore it to its initial
state of charge under specified conditions. Can be measured as ampere-hour,
voltage, or watt-hour efficiency.
- Electrolyte: The chemistry of a battery requires
a medium that provides the ion transport mechanism between the positive and
negative electrodes of a cell. This is often a water solution, but in lithium
batteries it consists of organic solvents with dissolved salts.
- Energy density (specific energy): These two
terms are often used interchangeably. Energy density refers mainly to
the ratio of a battery's available energy to its volume (watt hour/liter).
Specific energy refers to the ratio of energy to mass (watt hour/kg).
The energy is determined by the charge that can be stored and the cell voltage
(E=qV).
- Fuel cell: A cell in which one or both of the
reactants are not permanently contained in the cell, but are continuously
supplied from a source external to the cell and the reaction products
continuously removed. Unlike the metal anodes typically used in batteries, the
fuels in a fuel cell are usually gas or liquid, with oxygen as the oxidant. The
hydrogen/oxygen fuel cell is the most common. In this fuel cell, hydrogen is
oxidized at the anode:
| half-reaction |
V versus SHE |
| 2H2 > 4H+ +
4e- |
0 |
| 4H+ + O2 +
4e- > 2H2O |
1.2 |
Hydrogen/oxygen fuel cell systems
work well in space travel applications because of their high efficiency, high
power-to-weight and volume ratios, and usable reaction product (water). They
can function for many months as long as fuel is supplied and therefore the
energy density cannot be measured.
- Half-reaction: Refers to the chemical processes
occurring at each electrode. The potential of the two half-reactions add to
give us the overall cell potential. We can see this in the zinc mercury cell,
for example:
| Location |
Reaction |
Potential |
| Anode |
Zn + 2OH- > Zn(OH)2 +
2e- |
1.25 V |
| Cathode |
HgO +H2O + 2e- > Hg +
2OH- |
0.098 V |
| Overall |
Zn + HgO + H2O >
Zn(OH)2 + Hg |
1.35 V |
- Polarization: The voltage drop in a cell during
discharge due to the flow of an electrical current. The cell's internal
resistance increases with the buildup of a product of oxidation or a reduction
of an electrode, preventing further reaction.
- Power: Defined by voltage (V) and current (I),
P=VI.
Since V=IR, P=I2R and P=V2/R
Power also can be described by energy emitted per unit
of time: P=E/t.
Thus E=VIt=qV.
- Power density (specific power): Power
density is the ratio of the power available from a battery to its volume
(watts/liter). Specific power generally refers to the ratio of power to
mass (watts/kg). Comparison of power to cell mass is more common.
- Primary cells: A cell that is not designed for
recharging and is discarded once it has produced all its electrical
energy.
- Prismatic: Just a word to say that the cells are
not cylindrical, as nature intended battery cells to be, but fit nicely into a
parallelepiped, rectangular or any other such flattened shape.
- Peukart Effect: When a battery is discharged
extremely quickly it will have less capacity than expected. This is the Peukart
effect, which is very strong for lead acid batteries and many primary cells,
but much less so for nickel cadmium, NiMH and lithium batteries. Peukert's
equation is In · t = C, where I is the discharge rate, t is
the discharge time and C is the capacity. The exponent "n" depends on the
battery chemistry and the temperature. A log-log plot of discharge time versus
discharge load will have a slope of "n"
- Reserve cell: A primary cell that may be kept
inactive and which is activated by adding an electrolyte or electrode, or
melting an electrolyte which is normally in a solid state.
- Secondary cells: A cell capable of repeated use.
Its charge may be fully restored by passing an electric current through the
cell in the opposite direction to that of discharge, thus reversing the redox
reactions.

No one battery design is perfect for every application.
Choosing one requires compromise. That's why it's important to prioritize your
list of requirements. Decide which ones you absolutely must have and which you
can compromise on. Here are some of the parameters to consider:
- Voltage: Normal voltage during discharge,
maximum and minimum permissible voltages, discharge curve profile. Note that
secondary cells are characterized by their average voltage during a full
discharge, whereas primary cells are characterized by their peak voltage. Thus
an alkaline cells is rated at 1.5V, though its average voltage during discharge
is 1.2V. A NiMH cell is rated at 1.2 volts, but its discharge range is 1.4 to 1
volt.
- Duty cycle: Conditions the battery experiences
during use. Type of discharge and current drain, e.g.., continuous,
intermittent, continuous with pulses, etc. A 50% duty cycle means that the cell
is off for half the cycle, and on for the other half of the cycle.
- Temperature: In storage and in use. Temperatures
that are too high or too low can greatly reduce battery capacity.
- Shelf life: How rapidly the cell loses potential
while unused.
- Service life: Defined either in calendar time
or, for secondary cells, possible number of discharge/charge cycles, depending
on the battery application. Service life depends on battery design and
operational conditions, i.e., the stress put on a battery. For stationary and
motive power application, the end of service life is defined as the point at
which a battery's capacity drops to 80% of its original capacity. Exceptions
would include car batteries where the service life ends when the capacity falls
below 60%.
- Physical restrictions: These include dimensions,
weight, terminals, etc.
- Maintenance and resupply: Ease of battery
acquisition, replacement, charging facilities, disposal.
- Safety and reliability: Failure rates, freedom
from outgassing or leakage; use of toxic components; operation under hazardous
conditions; environmentally safe
- Cost: Initial cost, operating cost, use of
expensive materials
- Internal resistance: Batteries capable of a
high-rate discharge must have a low internal resistance.
- Specific energy: As discussed in the definition
section, this is a measurement of possible stored energy per kilogram of mass.
This number is purely theoretical as it does not take into account the mass of
inactive materials, nor the variation in chemical reactions.
- Specific power: Also defined in the definitions
section, a P=E/t, so the specific power is discussed at a specific discharge
rate. It is possible for batteries with a high specific energy to have a low
power density if they experience large voltage drops at high discharge rates.
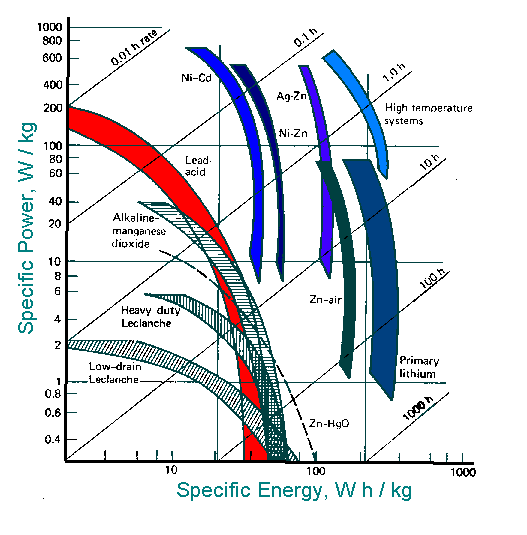
Specific power and specific energy can be compared in a Ragone plot .
- Unusual requirements: Very long-term or
extreme-temperature storage; very low failure rate; no voltage delay, etc.
Of course the ideal battery would perform well in all
these areas with a long shelf and service life, high specific energy and
specific power, low initial and maintenance costs, low environmental impact,
and good performance in a variety of conditions (temperatures, duty cycles,
etc.). When you find one that meets all these requirements, let us know! In the
meantime, we have to make do with batteries that work very well in specific
applications.
Primary Batteries
Leclanché
Cells(zinc carbon or dry
cell)
Anode: Zinc
Cathode: Manganese Dioxide (MnO2)
Electrolyte: Ammonium chloride or zinc chloride
dissolved in water
Applications: Flashlights, toys, moderate drain
use
The basic design of the Leclanché cell has been
around since the 1860s, and until World War II, was the only one in wide use.
It is still the most commonly used of all primary battery designs because of
its low cost, availability, and applicability in various situations. However,
because the Leclanché cell must be discharged intermittently for best
capacity, much of battery research in the last three decades has focused on
zinc-chloride cell systems, which have been found to perform better than the
Leclanché under heavier drain.
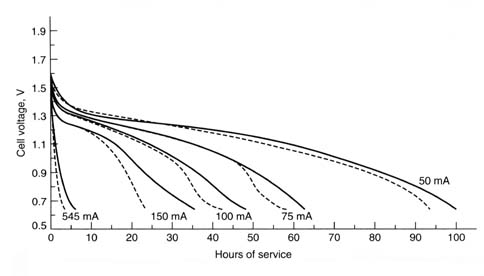
This figure shows typical discharge curves for
general-purpose Leclanché zinc chloride D-size cells discharge 2 H/day
at 20º C. Solid line—zinc chloride; broken
line—Leclanché (Linden 8.18). The zinc-chloride cell has a
higher service life and voltage than the Leclanché (at both higher and
lower discharge rates).
In an ordinary Leclanché cell the electrolyte
consists (in percent of atomic weight) of 26% NH4Cl (ammonium
chloride), 8.8% ZnCl2 (zinc chloride), and 65.2% water. The overall
cell reaction can be expressed:
Zn + 2MnO2 +2NH4Cl —> 2MnOOH
+ Zn(NH3)2Cl2 E=1.26
The electrolyte in a typical zinc chloride cell consists
of 15-40% ZnCl2 and 60-85% water, sometimes with a small amount of
NH4Cl for optimal performance. The overall cell reaction of the zinc
chloride as the electrolyte can be expressed:
Zn + 2MnO2 + 2H2O + ZnCl2
—> 2MnOOH + 2Zn(OH)Cl
MnO2, is only slightly conductive, so graphite
is added to improve conductivity. The cell voltage increases by using
synthetically produced manganese dioxide instead of that found naturally
(called pyrolusite). This does drive the cost up a bit, but it is still
inexpensive and environmentally friendly, making it a popular cathode.
These cells are the cheapest ones in wide use, but they
also have the lowest energy density and perform poorly under high-current
applications. Still, the zinc carbon design is reliable and more than adequate
for many everyday applications.

Anode: Zinc powder
Cathode: Manganese dioxide (MnO2)
powder
Electrolyte: Potassium hydroxide (KOH)
Applications: Radios, toys, photo-flash
applications, watches, high-drain applications
This cell design gets its name from its use of alkaline
aqueous solutions as electrolytes. Alkaline battery chemistry was first
introduced in the early ’60s. The alkaline cell has grown in popularity,
becoming the zinc-carbon cell's greatest competitor. Alkaline cells have many
acknowledged advantages over zinc-carbon, including a higher energy density,
longer shelf life, superior leakage resistance, better performance in both
continuous and intermittent duty cycles, and lower internal resistance, which
allows it to operate at high discharge rates over a wider temperature
range.
Zinc in a powdered form increases the surface area of the
anode, allowing more particle interaction. This lowers the internal resistance
and increases the power density. The cathode, MnO2, is synthetically
produced because of its superiority to naturally occurring MnO2.
This increases the energy density. Just as in the zinc carbon cell, graphite is
added to the cathode to increase conductivity. The electrolyte, KOH, allows
high ionic conductivity. Zinc oxide is often added to slow down corrosion of
the zinc anode. A cellulose derivative is thrown in as well as a gelling agent.
These materials make the alkaline cell more expensive than the zinc-carbon, but
its improved performance makes it more cost effective, especially in high drain
situations where the alkaline cell's energy density is much higher.
The half-reactions are:
Zn + 2 OH- —> ZnO + H2O + 2
e-
2 MnO2 + H2O + 2 e-
—>Mn2O3 + 2 OH-
The overall reaction is:
Zn + 2MnO2 —> ZnO +
Mn2O3 E=1.5 V
There are other cell designs that fit into the alkaline
cell category, including the mercury oxide, silver oxide, and zinc air cells.
Mercury and silver give even higher energy densities, but cost a lot more and
are being phased out through government regulations because of their high
toxicity as heavy metals. The mercury oxide, silver oxide, and zinc air (which
is being developed for electronic vehicles) are all discussed below.

Mercury Oxide
Cells
Anode: Zinc (or cadmium)
Cathode: Mercuric Oxide (HgO)
Electrolyte: Potassium hydroxide
Applications: Small electronic equipment, hearing
aids, photography, alarm systems, emergency beacons, detonators, radio
microphones
This is an obsolete technology. Most if not all of the
manufacture of these cells has been stopped by government regulators. Mercury
batteries come in two main varieties: zinc/mercuric oxide and cadmium/mercuric
oxide. The zinc/mercuric oxide system has high volumetric specific energy (400
Wh/L), long storage life, and stable voltage. The cadmium/mercuric oxide system
has good high temperature and good low temperature (-55 C to +80 C, some
designs to +180 C) and has very low gas evolution.
| Basic Cell Reaction |
Voltage |
Electrochemical
Efficiency |
| Zn + HgO = ZnO + Hg |
1.35 V |
820 mAH/g(Zn), 250 mAH/g(Hg) |
| Cd + HgO + H2O = Cd(OH2) + Hg |
0.91 V |
480 mAH/g(Cd) |
The electrolytes used in mercury
cells are sodium and/or potassium hydroxide solutions, making these alkaline
cells. These cells are not rechargeable.
 Zinc/Air
Cells
Zinc/Air
Cells
Anode: Amalgamated zinc powder and electrolyte
Cathode: Oxygen (O2)
Electrolyte: Potassium hydroxide (KOH)
Applications: Hearing aids, pagers, electric
vehicles
The zinc air cell fits into the alkaline cell category
because of its electrolyte. It also acts as a partial fuel cell because it uses
the O2 from air as the cathode. This cell is interesting technology,
even aside from the question "how do you use air for an electrode?" Actually,
oxygen is let in to the cathode through a hole in the battery and is reduced on
a carbon surface.
A number of battery chemistries involve a metal oxide and
zinc. The metal oxide reduces, the zinc becomes oxidized, and electric current
results. A familiar example is the old mercury oxide/zinc batteries used for
hearing aids. If you leave out the metal oxide you could double the capacity
per unit volume (roughly), but where would you get the oxygen? Right!
First let's look at the electrochemical reactions and find
that the open cell voltage should be 1.65 volts:
| Location |
Half Cell reactions |
Voltage |
| Anode |
Zn2+ + 2OH- —>
Zn(OH)2 |
1.25 |
| Cathode |
1/2 O2 + H2O + 2e —>
2 OH- |
0.4 |
| Overall |
2Zn +O2 +2H2O —>
2Zn(OH)2 |
1.65 |
The electrolyte is an alkali hydroxide in 20-40% weight
solution with water. One disadvantage is that since these hydroxides are
hygroscopic, they will pick up or lose water from the air depending on the
humidity. Both too little and too much humidity reduces the life of the cell.
Selective membranes can help. Oxygen from the air dissolves in the electrolyte
through a porous, hydrophobic electrode—a carbon-polymer or metal-polymer
composite.
Since there is no need to carry around the cathode, the
energy density of these batteries can be quite high, between 220–300 Wh/kg
(compared to 99–123 Wh/kg with a HgO cathode), although the power density
remains low. However, the use of potassium or sodium hydroxides as the
electrolyte is a problem, since these can react with carbon dioxide in the air
to form alkali carbonates. For this reason large zinc air batteries usually
contain a higher volume of CO2 absorbing material (calcium oxide
flake) than battery components. This can cancel out the huge increase in energy
density gained by using the air electrode.
This cell has the additional benefits of being
environmentally friendly at a relatively low cost.
These batteries can last indefinitely before they are
activated by exposing them to air, after which they have a short shelf life.
For this reason (as well as the high energy density) most zinc-air batteries
are used in hearing aids. There is a company promoting them for use in electric
vehicles also because they are environmentally friendly and cost relatively
little. The idea is to have refueling stations where the zinc oxide waste can
be replaced by fresh zinc pellets.

Aluminum Air
Cells
Although, to our way of thinking, the metal/air batteries
are strictly primary, cells have been designed to have the metal replaceable.
These are called mechanically rechargeable batteries. Aluminum/air is an
example of such a cell. Aluminum is attractive for such cells because it is
highly reactive, the aluminum oxide protective layer is dissolved by hydroxide
electrolytes, and it has a nice, high voltage. The overall chemical reaction
is:
| Location |
Half Cell reactions |
Voltage |
| Anode |
Al + 4 OH-—>
Al(OH)4- + 3e |
-2.35 |
| Cathode |
3/4 O2 + 3/2 H2O + 3e—>
3OH- |
0.40 |
| Overall |
Al + 3/2 HO + 3/4 O2 —>
Al(OH)3 |
2.75 V |
As I mentioned above, alkali (chiefly potassium hydroxide)
electrolytes are used, but so also are neutral salt solutions. The alkali cell
has some problem with the air electrode, because the hydroxide ion makes a gel
in the porous electrode, polarizing it. The typical aluminum hydroxide gel is a
problem on either electrode because it sucks up a lot of water. Using a
concentrated caustic solution prevents this, but is very reactive with the
aluminum electrode, producing hydrogen gas. Another way to prevent the gel
formation is to seed the electrolyte with aluminum trihydroxide crystals. These
act to convert the aluminum hydroxide to aluminum trihydroxide as the crystals
grow. To prevent hydrogen gas evolution tin and zinc have been used as
corrosion inhibitors. A number of additives are used to control the reactions.
A disadvantage of the alkaline electrolyte is that it reacts with atmospheric
carbon dioxide.
Aluminum / air cells have also been made for marine
applications. These are "rechargeable" by replacing the seawater electrolyte
until the aluminum is exhausted, then replacing the aluminum. Some cells that
are open to seawater have also been researched. Since salt water solutions tend
to passivate the aluminum, pumping the electrolyte back and forth along the
cell surface has been successful. For those cells that don't need to use ocean
water, an electrolyte of KCL and KF solutions is used.
Air electrodes of Teflon-bonded carbon are used without a
catalyst.
 v v
Lithium
Cells
Applications: Pacemakers, defibrillators, watches,
meters, cameras, calculators, portable, low-power use
Lithium battery chemistry comprise a number of cell
designs that use lithium as the anode. Lithium is gaining a lot of popularity
as an anode for a number of reasons. In this comparison of anode materials, we
can see some reasons why:
| Anode |
Atomic
mass (g) |
Standard
potential (V) |
Density
g/cm3 |
Melting
point ºC |
Electrochemical
Equivalence
(Ah/g) |
| Li |
6.94 |
3.05 |
0.54 |
180 |
3.86 |
| Na |
23.0 |
2.7 |
0.97 |
97.8 |
1.16 |
| Mg |
24.3 |
2.4 |
1.74 |
650 |
2.20 |
| Al |
26.9 |
1.7 |
2.7 |
659 |
2.98 |
| Ca |
40.1 |
2.87 |
1.54 |
851 |
1.34 |
| Fe |
55.8 |
0.44 |
7.85 |
1528 |
0.96 |
| Zn |
65.4 |
0.76 |
7.1 |
419 |
0.82 |
| Cd |
112 |
0.40 |
8.65 |
321 |
0.48 |
| Pb |
207 |
0.13 |
11.3 |
327 |
0.26 |
Note that lithium, the lightest of the metals, also has
the highest standard potential of all the metals, at over 3 V. Some of the
lithium cell designs have a voltage of nearly 4 V. This means that lithium has
the highest energy density. Many different lithium cells exist because of its
stability and low reactivity with a number of cathodes and non-aqueous
electrolytes. The most common electrolytes are organic liquids with the notable
exceptions of SOCl2 (thionyl chloride) and
SO2Cl2 (sulfuryl chloride). Solutes are added to the
electrolytes to increase conductivity.
Lithium cells have only recently become commercially
viable because lithium reacts violently with water, as well as nitrogen in air.
This requires sealed cells. High-rate lithium cells can build up pressure if
they short circuit and cause the temperature and pressure to rise. Thus, the
cell design needs to include weak points, or safety vents, which rupture at a
certain pressure to prevent explosion.
Lithium cells can be grouped into three general
categories: liquid cathode, solid cathode, and solid electrolyte. Let's look at
some specific lithium cell designs within the context of these three
categories.
 v v
Liquid cathode lithium cells:
These cells tend to offer higher discharge rates because
the reactions occur at the cathode surface. In a solid cathode, the reactions
take longer because the lithium ions must enter into the cathode for discharge
to occur. The direct contact between the liquid cathode and the lithium forms a
film over the lithium, called the solid electrolyte interface (SEI). This
prevents further chemical reaction when not in use, thus preserving the cell's
shelf life. One drawback, though, is that if the film is too thick, it causes
an initial voltage delay. Usually, water contamination is the reason for the
thicker film, so quality control is important.
 LiSO2
Lithium–Sulfur Dioxide LiSO2
Lithium–Sulfur Dioxide
This cell performs very well in high current applications
as well as in low temperatures. It has an open voltage of almost 3 V and a
typical energy density of 240–280 Wh/kg. It uses a cathode of porous
carbon with sulfur dioxide taking part in the reaction at the cathode. The
electrolyte consists of an acetonitrile solvent and a lithium bromide solute.
Polypropylene acts as a separator. Lithium and sulfur dioxide combine to form
lithium dithionite:
2Li + 2SO2 —>
Li2S2O4
These cells are mainly used in military applications for
communication because of high cost and safety concerns in high-discharge
situations, i.e., pressure buildup and overheating.
 LiSOCl2 Lithium Thionyl Chloride LiSOCl2 Lithium Thionyl Chloride
This cell consists of a high-surface area carbon cathode,
a non-woven glass separator, and thionyl chloride, which doubles as the
electrolyte solvent and the active cathode material. Lithium aluminum chloride
(LiAlCl4) acts as the electrolyte salt.
The materials react as follows:
| Location |
Reaction |
| Anode |
Li —> Li+ + e- |
| Cathode |
4Li+ + 4e- +
2SOCl2 —> 4LiCl + SO2 + S |
| Overall |
4Li + 2SOCl2 —> 4LiCl +
SO2 + S |
During discharge the anode gives off lithium ions. On the
carbon surface, the thionyl chloride reduces to chloride ions, sulfur dioxide,
and sulfur. The lithium and chloride ions then form lithium chloride. Once the
lithium chloride has deposited at a site on the carbon surface, that site is
rendered inactive. The sulfur and sulfur dioxide dissolve in the electrolyte,
but at higher-rate discharges SO2 will increase the cell
pressure.
This system has a very high energy density (about 500
Wh/kg) and an operating voltage of 3.3–3.5 V. The cell is generally a
low-pressure system
In high-rate discharge, the voltage delay is more
pronounced and the pressure increases as mentioned before. Low-rate cells are
used commercially for small electronics and memory backup. High-rate cells are
used mainly for military applications.

Solid cathode lithium cells:
These cells cannot be used in high-drain applications and
don't perform as well as the liquid cathode cells in low temperatures. However,
they don't have the same voltage delay and the cells don't require
pressurization. They are used generally for memory backup, watches, portable
electronic devices, etc.
 LiMnO2 LiMnO2
These account for about 80% of all primary lithium cells,
one reason being their low cost. The cathode used is a heat-treated
MnO2 and the electrolyte a mixture of propylene carbonate and
1,2-dimethoyethane. The half reactions are
| Anode |
Li —> Li+ + e |
| Cathode |
MnIVO2 + Li+ + e
—> MnIIIO2(Li+) |
| Overall |
Li + MnIVO2 —>
MnIIIO2(Li+) |
At lower temperatures and in high-rate discharge, the
LiSO2 cell performs much better than the LiMnO2 cell. At
low-rate discharge and higher temperatures, the two cells perform equally well,
but LiMnO2 cell has the advantage because it doesn't require
pressurization.
 Li(CF)n
Lithium polycarbon monofluoride Li(CF)n
Lithium polycarbon monofluoride
These cells are used in coin cells for watches and
memory-back up, nuclear missile batteries, the space shuttle safety system, and
other governmental and space applications. The cathode in this cell is carbon
monofluoride, a compound formed through high-temperature intercalation. This is
the process where foreign atoms (in this case fluorine gas) incorporate
themselves into some crystal lattice (graphite powder), with the crystal
lattice atoms retaining their positions relative to one another. This is not a
stoichiometric reaction, so the proportion of fluorine atoms can vary between
0.8 and 1.2, which is why the half-reactions are also not stoichiometric. This
is interesting because it allows a lithium-fluorine reaction, which is probably
the most energetic possible by safely storing the fluorine atoms in a graphite
matrix. This is similar to how lithium ions are stored in lithium ion
batteries. The carbon intercalation makes it safe, but it also reduces the
voltage and lowers the electrical current capability.
A typical electrolyte is lithium tetrafluoroborate
(LiBF4) salt in a solution of propylene carbonate (PC) and
dimethoxyethane (DME).
| Anode |
Li —> Li+ + e |
| Cathode |
CFx + xe —> xC +
xF |
| Overall |
CFx + xLi —>
xLiF+ xC |
Note that one of the reaction products is carbon, which
lowers the resistance of the cell as the battery is discharged. These cells
also have a high voltage (about 3.0 V open voltage) and a high energy density
(around 250 Wh/kg). All this and a 7-year shelf life makes them very suitable
for low- to moderate-drain use, e.g., watches, calculators, and memory
applications.
 v v
Solid electrolyte lithium cells:
All commercially manufactured cells that use a solid
electrolyte have a lithium anode. They perform best in low-current applications
and have a very long service life. For this reason, they are used in
pacemakers
 LiI2—Lithium iodine cells use solid LiI as their
electrolyte and also produce LiI as the cell discharges. The cathode is
poly-2-vinylpyridine (P2VP) with the following reactions: LiI2—Lithium iodine cells use solid LiI as their
electrolyte and also produce LiI as the cell discharges. The cathode is
poly-2-vinylpyridine (P2VP) with the following reactions:
| Anode |
2Li —> 2Li+ + 2e |
| Cathode |
2Li+ + 2e + P2VP· nI2 —> P2VP· (n–1)I2 + 2LiI |
| Overall |
2Li + P2VP·
nI2 —> P2VP·
(n–1)I2 +2LiI |
LiI is formed in situ by direct reaction of the
electrodes.

Lithium-Iron Cells
The Lithium-Iron
chemistry deserves a separate section because it is one of a handful of lithium
metal systems that have a 1.5 volt output (others are lithium/lead bismuthate,
lithium/bismuth trioxide, lithium/copper oxide, and lithium/copper sulfide).
Recently consumer cells that use the Li/Fe have reached the market, including
the Energizer. These have advantage of having the same voltage as alkaline
batteries with much more energy storage capacity, so they are called "voltage
compatible" lithiums. They are not rechargeable. They have about 2.5 times the
capacity of an alkaline battery of the same size, but only under high current
discharge conditions (digital cameras, flashlights, motor driven toys, etc.).
For small currents they don't have any advantage. Another advantage is the low
self-discharge rate–10 years storage is quoted by the manufacturer. The
discharge reactions are:
| Type |
Reaction |
Nominal Voltage |
Range |
| FeS2 Version |
2 FeS2 + 4 Li —> Fe +
2Li2S |
1.6 Volts |
1.6-1.4 V |
| FeS Version |
FeS + 2Li —> Fe + Li2S |
1.5 Volts |
1.5-1.2 V |
Both Iron sulfide and Iron disulfide are used, the
FeS2 is used in the Energizer. Electrolytes are organic materials such as
propylene carbonate, dioxolane and dimethoxyelthane

Magnesium-Copper Chloride Reserve Cells
The
magnesium-cuprous chloride system is a member of the reserve cell family. It
can't be used as a primary battery because of its high self-discharge rate, but
it has a high discharge rate and power density, so it can be made "dry charged"
and sit forever ready, just add water. The added advantage of being
light-weight has made these practical for portable emergency
batteries.
It works by depositing copper metal out onto the magnesium
anode, just like the old copper-coated nail experiment.
Variations of
this battery use silver chloride, lead chloride, copper iodide, or copper
thiocyanate to react with the magnesium.
The water does not have to be
pure, sea water, tap water, or even bio-derived waste fluids have been used.
The torpedo batteries force seawater through the battery to get up to 460 kW of
power to drive the propeller.
| Type |
Reaction |
Nominal Voltage |
Range |
| Mg CuCl |
Mg + 2 CuCl —> MgCl2+ 2 Cu |
1.6 Volts |
1.5-1.6V |
Secondary batteries
Anode: Sponge metallic lead
Cathode: Lead dioxide (PbO2)
Electrolyte: Dilute mixture of aqueous sulfuric
acid
Applications: Motive power in cars, trucks,
forklifts, construction equipment, recreational water craft, standby/backup
systems
Used mainly for engine batteries, these cells represent
over half of all battery sales. Some advantages are their low cost, long life
cycle, and ability to withstand mistreatment. They also perform well in high
and low temperatures and in high-drain applications. The chemistry lead acid
battery half-cell reactions are:
| half-reaction |
V vs SHE |
| Pb + SO42- —>
PbSO4 + 2e- |
.356 |
| PbO2 + SO42- +
4H+ + 2e- —> PbSO4 +
2H2O |
1.685 |
There are a few problems with this design. If the cell
voltages exceed 2.39 V, the water breaks down into hydrogen and oxygen (this
so-called gassing voltage is temperature dependent, for a chart of the
temperature dependence click here  ). This requires replacing the cell's water. Also, as the
hydrogen and oxygen vent from the cell, too high a concentration of this
mixture will cause an explosion. Another problem arising from this system is
that fumes from the acid or hydroxide solution may have a corrosive effect on
the area surrounding the battery. ). This requires replacing the cell's water. Also, as the
hydrogen and oxygen vent from the cell, too high a concentration of this
mixture will cause an explosion. Another problem arising from this system is
that fumes from the acid or hydroxide solution may have a corrosive effect on
the area surrounding the battery.
These problems are mostly solved by sealed cells, made
commercially available in the 1970s. In the case of lead acid cells, the term
"valve-regulated cells" is more accurate, because they cannot be sealed
completely. If they were, the hydrogen gas would cause the pressure to build up
beyond safe limits. Catalytic gas re-combiners do a great deal to alleviate
this problem. They convert the hydrogen and oxygen back into water, achieving
about 85% efficiency at best. Although this doesn't entirely eliminate the
hydrogen and oxygen gas, the water lost becomes so insignificant that no refill
is needed for the life of the battery. For this reason , these cells are often
referred to as maintenance-free batteries. Also, this cell design prevents
corrosive fumes from escaping.
These cells have a low cycle life, a quick self discharge,
and low energy densities (normally between 30 and 40 Wh/kg). However, with a
nominal voltage of 2 V and power densities of up to 600 W/kg, the lead-acid
cell is an adequate, if not perfect, design for car batteries.

Anode: Cadmium
Cathode: Nickel oxyhydroxide Ni(OH)2
Electrolyte: Aqueous potassium hydroxide (KOH)
Applications: Calculators, digital cameras, pagers,
lap tops, tape recorders, flashlights, medical devices (e.g., defibrillators),
electric vehicles, space applications
The cathode is nickel-plated, woven mesh, and the anode is
a cadmium-plated net. Since the cadmium is just a coating, this cell's negative
environmental impact is often exaggerated. (Incidentally, cadmium is also used
in TV tubes, some semiconductors, and as an orange-yellow dye for plastics.)
The electrolyte, KOH, acts only as an ion conductor and does not contribute
significantly to the cell's reaction. That's why not much electrolyte is
needed, so this keeps the weight down. (NaOH is sometimes used as an
electrolyte, which doesn't conduct as well, but also doesn't tend to leak out
of the seal as much). Here are the cell reactions:
| Reaction |
V vs SHE |
| Cd + 2OH- —> Cd(OH)2
+ 2e- |
0.81 |
| NiO2 + 2H2O + 2e-
—> Ni(OH)2 + 2OH- |
0.49 |
| Cd +NiO2 + 2H2O —>
Cd(OH)2 + Ni(OH)2 |
1.30 |
Advantages include good performance in high-discharge and
low-temperature applications. They also have long shelf and use life.
Disadvantages are that they cost more than the lead-acid battery and have lower
power densities. Possibly its most well-known limitation is a memory effect,
where the cell retains the characteristics of the previous cycle.
This term refers to a temporary loss of cell capacity,
which occurs when a cell is recharged without being fully discharged. This can
cause cadmium hydroxide to passivate the electrode, or the battery to wear out.
In the former case, a few cycles of discharging and charging the cell will help
correct the problem, but may shorten the lifetime of the battery. The true
memory effect comes from experience with a certain style of NiCad in space use,
which were cycled within a few percent of discharge each time.
An important thing to know about "conditioning " a NiCd
battery is that the deep discharge spoken of is not a discharge to zero volts,
but to about 1 volt per cell.
 v v
Anode:Hydrogen Gas
Cathode: Nickel oxyhydroxide
Electrolyte: Potassium hydroxide
Applications:Space satellites that require long
cycle life, over 40,000 cycles. Nickel/Hydrogen batteries have a high
self-discharge rate, something like 80% a month, which isn't a problem for
satellite applications.
The NiH2 cell is a welded pressure vessel. It
has a high specific energy, 60WH/kg, long life, can tolerate overcharge and
cell reversal, but has a low volumetric energy density, 50 WH/liter.
Here are the cell reactions:
| Location |
Reactions |
Voltage |
| Anode |
½H2 + OH- —>
H2O + e- |
0.83 |
| Cathode |
NiOOH + H2O + e- —>
Ni(OH)2 + OH- |
0.52 |
| Overall |
NiOOH + ½H2 —>
Ni(OH)2 |
1.35 |
In order to get the hydrogen gas into solution a
Teflon-bonded platinum black catalyst is used, similar to that used in fuel
cells. This platinum electrode has the added advantage that it can recombine
oxygen with hydrogen extremely fast. Since the only bad chemical reaction
during over charge is the creation of oxygen at the positive electrode this
means that the Nickel/Hydrogen battery is impossible to overcharge (though
there may be a thermal runaway problem if the excess heat isn't dissipated.) A
similar reaction keeps any damage from being done if the cell is
reverse-charged.
The battery weight for a 10kW satellite is about 350 kg,
or 770 lbs.
Anode: Rare-earth or nickel alloys with many
metals
Cathode: Nickel oxyhydroxide
Electrolyte: Potassium hydroxide
Applications: Cellular phones, camcorders,
emergency backup lighting, power tools, laptops, portable, electric
vehicles
This sealed cell is a hybrid of the NiCd and
NiH2 cells. Previously, this battery was not available for
commercial use because, although hydrogen has wonderful anodic qualities, it
requires cell pressurization. Fortunately, in the late 1960s scientists
discovered that some metal alloys (hydrides such as LiNi5 or
ZrNi2) could store hydrogen atoms, which then could participate in
reversible chemical reactions. In modern NiMH batteries, the anode consists of
many metals alloys, including V, Ti, Zr, Ni, Cr, Co, and Fe.
Except for the anode, the NiMH cell very closely resembles
the NiCd cell in construction. Even the voltage is virtually identical, at 1.2
volts, making the cells interchangeable in many applications. Here are the cell
reactions:
| Location |
Reactions |
Voltage |
| Anode |
MH + OH- —> M + H2O +
e- |
0.83 |
| Cathode |
NiOOH + H2O + e- —>
Ni(OH)2 + OH- |
0.52 |
| Overall |
NiOOH + MH —> Ni(OH)2 + M |
1.35 |
The anodes used in these cells are complex alloys
containing many metals, such as an alloy of V, Ti, Zr, Ni, Cr, Co, and
(!) Fe. The underlying chemistry of these alloys and reasons for superior
performance are not clearly understood, and the compositions are determined by
empirical testing methods.
A very interesting fact about these alloys is that some
metals absorb heat when absorbiong hydrogen, and some give off heat when
absorbing hydrogen. Both of these are bad for a battery, since we would like
the hydregen to move easily in and out without any energy transfer. The
successful alloys are all mixtures of exothermic and endothermic metals to
achieve this.
Hydrogen Storage Metals
Comparison:
| Material |
Density |
H2 Storage Capacity |
| LaNi5 |
8.3 |
0.11 g/cc |
| FeTi |
6.2 |
0.11 |
| Mg2Ni |
4.1 |
0.15 |
| Mg |
1.74 |
0.13 |
| MgNi Eutectic |
2.54 |
0.16 |
| liquid H2 |
0.07 |
0.07 |
Please notice that the density of hydrogen stored in a
metal hydride is higher than that of pure liquid hydrogen! Commercial NiMH
batteries are mostly of the rare earth-nickel type, of which LaNi5
is a representative. These alloys can store six hydrogen atoms per unit cell
such as LaNi5H6. Even misch metal nickel alloys are used
to save the cost of separation.
The electrolyte of commercial NiMH batteries is typically
6 M KOH
The NiMH cell does cost more and has half the service life
of the NiCd cell, but it also has 30% more capacity, increased power density
(theoretically 50% more, practically 25% more). The memory effect, which was at
one time thought to be absent from NiMH cells, is present if the cells are
treated just right. To avoid the memory effect fully discharge once every 30 or
so cycles. There is no clear winner between the two. The better battery depends
on what characteristics are more crucial for a specific application.
 v v
Anode: Molten sodium
Cathode: Molten sulfur
Electrolyte: Solid ceramic beta alumina
(ß"-Al2O3)
Applications: Electric vehicles, aerospace
(satellites)
This cell have been studied extensively for electric
vehicles because of its inexpensive materials, high cycle life, and high
specific energy and power. Specific energies have reached levels of 150 W-h/kg
and specific powers of 200 W/kg. The half-reactions are:
| half-reaction |
V vs SHE |
| 2Na —> 2Na+ +
2e- |
|
| 3S + 2e- —>
S32- |
|
2Na + 3S —> Na2S3 2.076
V
Despite these advantages there are couple of disadvantages
serious enough that other alternatives, such as lithium-ion, nickel-metal
hydride, and lithium polymer, have emerged as the most promising solutions to
electric vehicle power. One is that the power output is very small at room
temperature. The temperature must be kept at around 350 ºC to keep the
sulfur in liquid form and to be effective in motive power applications. This is
achieved through insulation or heating through the cells own power. This lowers
the energy density.
The second problem has to do with electrolyte breakdown,
which is one of the principal causes of sodium sulfur cell failure. The
electrolyte, ceramic beta"-alumina, has several attractive characteristics. It
has all the benefits of a solid electrolyte with the added qualities of a high
ionic conductivity with a small electronic transfer, all with the added benefit
of being a solid. However, ceramic beta"-alumina also is brittle and develops
microfissures. Thus the liquid sodium and sulfur come in contact—with
explosively violent results.
Recently, some research efforts have focussed on replacing
the molten sulfur cathode with a poly(disulfide) such as
poly(ethylenedisulfide), (SSCH2CH2)n. These
cells can be discharged just above the melting temperature of Na (90 °C).
The net cell reaction becomes:
2 Na + (SSR)n=Na2SSR
where the discharge reaction involves scission of the S-S
disulfide linkage in the polymer backbone, and charge involves repolymerization
of the resulting dithiolate salt.
One of these is the sodium/metal chloride, which in
addition to beta"-alumina has a secondary electrolyte (NaAlCl4) to
conduct ions from the first electrolyte to the cathode. This is necessary
because the metal chloride is a solid.
 v v
Nickel/Sodium Cells
These are specialty cells
made by one manufacturer in England, Beta Research. They have advantages for
electric vehicles. The cell runs hot, about 300 degrees C, but this isn't a
worry, since they heat themselves up during discharge. The discharge reaction
is:
| Location |
Half Reaction |
Voltage |
| Charge |
2 NaCl + Ni Z —> 2Na +NiCl2 |
|
| Discharge |
NiCl2 + 2 Na —> Ni + 2NaCl |
2.58 V |
The electrolyte on the nickel side of the alumina
separator is sodium tetracloroaluminate.NaAlCl4, which melts at 151
degrees C.. Energy density is 100 to 150 Wh/kg. These use an aluminum oxide
ceramic as a separator, similar to that of the sodium-sulfur cell. They have
the same danger of rupture of the separator, but have a unique solution to the
problem. The cell is encased in a two-wall steel thermally insulated package.
If the separator breaks the energy is confined within this package. A cell that
is broken in this way has a low resistance, so it can continue to reside in the
battery pack without causing a vehicle break-down. This double-insulated case
also prevents the cell from spilling in car crashes.
There are no
higher-voltage reactions or other side reactions, so the inventors claim that
up to the point of full charge the cell is 100% coulomb efficient–meaning
that the amp-hours you put in is exactly the same as the amp-hours you get out.
Overcharging does not damage the cell, so the battery packs are easy to keep in
balance–just overcharge the whole pack.
It seems that the cell has
no self-discharge if the batteries are cold, (solid blocks of sodium don't
migrate at room temperature) and that a pack requires about 24 hours to get to
temperature with a 230 VAC input to the pack heater.

Anode: Carbon compound, graphite
Cathode: Lithium oxide
Electrolyte:
Applications: Laptops, cellular phones, electric
vehicles
Lithium batteries that use lithium metal
have safety disadvantages when used as secondary (rechargeable) energy sources.
For this reason a series of cell chemistries have been developed using lithium
compounds instead of lithium metal. These are called generically Lithium ion
Batteries.
Cathodes consist of a a layered crystal (graphite) into
which the lithium is intercalated. Experimental cells have also used lithiated
metal oxide such as LiCoO2,
NiNi0.3Co0.7O2, LiNiO2,
LiV2O5, LiV6O13,
LiMn4O9, LiMn2O4,
LiNiO0.2CoO2.
Electrolytes are usually LiPF6, although this
has a problem with aluminum corrosion, and so alternatives are being sought.
One such is LiBF4. The electrolyte in current production batteries
is liquid, and uses an organic solvent.
Membranes are necessary to separate the electrons from the
ions. Currently the batteries in wide use have microporous polyethylene
membranes.
Intercalation (rhymes with relation—not inter-cal,
but in-tercal-ation) is a long-studied process which has finally found a
practical use. It has long been known that small ions (such as lithium, sodium,
and the other alkali metals) can fit in the interstitial spaces in a graphite
crystal. Not only that, but these metallic atoms can go farther and force the
graphitic planes apart to fit two, three, or more layers of metallic atoms
between the carbon sheets. You can imagine what a great way this is to store
lithium in a battery—the graphite is conductive, dilutes the lithium for
safety, is reasonably cheap, and does not allow dendrites or other unwanted
crystal structures to form.
 v v
Manganese-Titanium (Lithium) Cells
Anode: Lithium-Titanium Oxide
Cathode: Lithium intercalated Manganese Dioxide
Electrolyte:
Applications: Watches, other ultra-low discharge
applications
This technology might be called Manganese-Titanium, but it
is just another lithium coin cell. It has "compatible" voltage – 1.5 V to
1.2 Volts, like the Lithium-Iron cell, which makes it convenient for
applications that formerly used primary coin cells. It is unusual for a lithium
based cell because it can withstand a continuous overcharge at 1.6 to 2.6 volts
without damage. Although rated for 500 full discharge cycles, it only has a 10%
a year self-discharge rate, and so is used in solar charged watches with
expected life of 15+ years with shallow discharging. The amp-hour capacity and
available current output of these cells is extremely meager. The range of
capacities from Panasonic is 0.9 to 14 mAH (yes, 0.9 milliamp hours). The
maximum continuous drain current is 0.1 to 0.5 mA.
 v v
Rechargeable Alkaline
Manganese Cells
Anode: Zinc
Cathode:Manganese Dioxide
Electrolyte: Potassium Hydroxide Solution
Applications: Consumer devices
Yes, this is the familiar alkaline battery, but specially
designed to be rechargeable, and with a hot new acronym—RAM (haven't I
seen that acronym somewhere before?). In the charging process, direct-current
electrical power is used to reform the active chemicals of the battery system
to their high-energy charge state. In the case of the RAM battery, this
involves oxidation of manganese oxyhydroxide (MnOOH) in the discharged positive
electrode to manganese dioxide (MnO2), and of zinc oxide (ZnO) in the negative
electrode to metallic zinc.
Care must be taken not to overcharge to prevent
electrolysis of the KOH solution electrolyte, or to charge at voltages higher
than 1.65 V (depending on temperature) to avoid the formation of higher oxides
of manganese.

Nickel Zinc
Cells
Anode: Zinc
Cathode: Nickel oxide
Electrolyte: Potassium hydroxide
Applications:Electric vehicles, standby load
service
The
combination of nickel and zinc is very interesting because of the low cost and
low toxicity of the constituents. There have been many technical obstacles, but
a string of recent patents and a commercial start-up based on a KOH electrolyte
holds great promise for applications where light weight is an issue.
The nickel/zinc battery uses zinc as the negative
electrode and nickel hydroxide as the positive. The discharge reactions
are:
| Location |
Half Reaction |
Voltage |
| Anode |
Zn + 2OH- —> Zn(OH)2+
2e |
1.24 V |
| Cathode |
2NiOOH + 2H2O —>
2Ni(OH)2 + 2OH- |
0.49 V |
| Overall |
2NiOOH + Zn + 2H2O —>
2Ni(OH)2 + Zn(OH)2 |
1.73 |
These cells run between 1.55 and 1.65 V. Theoretical
energy density is 334 Wh/kg, or about 1.3 kg of nickel and 0.7 kg of zinc per
kilowatt-hour. The internal resistance of nickel/zinc batteries is remarkably
low, which makes this system particularly attractive for high charge and
discharge rates
Practical specific energy is around 60 Wh/kg. The
technical problems that have plagued these batteries so far are dissolution of
the zinc in the electrolyte, and uneven redepositing of the zinc during
charging. Progress in these batteries has been mostly in the improvement of the
zinc electrode. The charging is tricky because the termination voltage is a
strong function of temperature.
 v v
Iron Nickel
Cells
Anode: Iron
Cathode: Nickel oxyhydroxide
Electrolyte: Potassium hydroxide
Applications:
This battery was introduced by Thomas
Edison. It is a very robust battery: it can withstand overcharge,
overdischarge, and remaining discharged for long periods of time without
damage. It is good for high depths of discharge and can have very long life
even if so treated. It has low energy density, a high self-discharge rate, and
evolves hydrogen during both charge and discharge. It is often used in backup
situations where it can be continuously charged and can last for 20 years.
The chemistry involves the movement of oxygen from one
electrode to the other: 3Fe + 8NiOOH + 4H2O=8 Ni(OH)2 +Fe3O4.
| Half Reaction |
Voltage |
| Fe + 2OH- —> Fe(OH)2
+2e- |
|
| 3Fe(OH)2 + 2OH- —>
Fe3O4 + 4H2O + 2e- |
|
The open circuit voltage of this system is
1.4 V, and the discharge voltage is about 1.2 V. The electrolyte is 30% KOH
solution, with some additives.
The ability of this system to survive
frequent cycling is due to the low solubility of the reactants in the
electrolyte. The formation of metallic iron on charge is slow because of the
low solubility of the Fe3O4, which is good and bad. It is
good because the slow formation of iron crystals preserves the electrode
morphology. It is bad because it limits the high rate performance: these cells
take a charge slowly, and give it up slowly.
 v v
Iron Air
Cells
The Iron/Air is another of the air-electrode
batteries. The electrochemistry is as follows:
| Half Reaction |
Voltage |
| O2 + 2Fe +2H2O=2Fe(OH)2 |
|
| O2 +2H2O +2e=H2O2 +2(OH) |
|
These batteries require a high degree of
support, since the CO2 must be taken out of the air in order to
prevent potassium carbonates forming in the KOH electrolyte. They have been
built in large backup systems. The air electrode consists of a catalyst on a
support. For example a carbon particle substrate held together with Teflon,
coated with a silver complex catalyst. Support is provided by a silver-plated
nickel screen.
 v v
Iron Silver
Cells
These have a very high energy density, and a
good cycle life. It is an alkaline battery with a KOH electrolyte, and the
working materials are silver oxide and metallic iron. The high cost of these
batteries have long been a problem, but an ounce of silver in a cell phone
battery would probably cost less than an ounce of the rare earths now used in
some NiMH batteries.
 v v
Redox (Liquid
Electrode) Cells
These consist of a semipermeable membrane having different
liquids on either side. The membrane permits ion flow but prevents mixing of
the liquids. Electrical contact is made through inert conductors in the
liquids. As the ions flow across the membrane an electric current is induced in
the conductors. These cells and batteries have two ways of recharging. The
first is the traditional way of running current backwards. The other is
replacing the liquids, which can be recharged in another cell. A small cell can
also be used to charge a great quantity of liquid, which is stored outside the
cells. This is an interesting way to store energy for alternative energy
sources that are unreliable, such as solar, wind, and tide. These batteries
have low volumetric efficiency, but are reliable and very long lived.
Electrochemical systems that can be used are
FeCl3 (cathode) and TiCl3 or CrCl2
(anode). Vanadium redox cells: A particularly interesting cell uses vanadium
oxides of different oxidation states as the anode and cathode. These solutions
will not be spoiled if the membrane leaks, since the mixture can be charged as
either reducing or oxidizing components.

Unlike batteries, which store energy chemically,
capacitors store energy as an electrostatic field. Typically, a battery is
known for storing a lot of energy and little power; a capacitor can provide
large amounts of power, but low amounts of energy. A capacitor is made of two
conducting plates and an insulator called the dielectric, which conducts
ionically, but not electrically. In a capacitor,
Ecap = qV = ½CV2
where the capacitance, C, is directly proportional to the
surface area of the plates and inversely proportional to the distance between
them.
So in other words, as the plate surface area increases and
the distance between the plates decreases, the energy you can store in a
capacitor increases. Normal every-day capacitors have capacity on the orders of
millifarads per cubic foot. Aluminum electrolytics are about a farad per cubic
foot. But for useful energy storage we need farads per cubic inch. That is
where supercapacitors come in.
First let's see how clever we can get to obtain a big
surface area in a small volume. Imagine a polymer foam cleaning sponge. It has
a tremendous amount of surface area in a small area because of all the
crenulations (OK, nooks and crannies). Now, put it in a furnace, excluding the
oxygen and bake it until only the carbon is left. You now have a conductive
carbon surface with an incredible surface area in a small volume.
But to get a high capacitance there has to be two plates.
You can't just go in there and create complimentary surface as the other
electrode—or can you? Yes, just fill it with a conductive liquid (e.g., an
aqueous acid or salt solution). The last thing you need is an ultra-thin
insulator on the carbon. Ultra thin to get high capacitance, and insulator so
the carbon and the liquid don't short out. This is also easy, you can
electrochemically deposit an insulator on the carbon surface (or
electrochemically deposit something that could be turned into an insulator upon
baking).
Now attach one electrode to the carbon, one to the liquid,
and you can have a capacitor that can have Farads of capacitance per cubic
inch. Very nice.
Most practical supercapacitors have low voltage (2 to 5
V—remember that insulator is ultra-thin and so can break down at low
voltages), which is a problem for energy storage, since the stored energy is
proportional to the square of the voltage. Also, conduction through an ionic
liquid is slow, so these capacitors cannot be discharged quickly compared with
standard capacitors, but can be discharged very quickly compared to
batteries!
Typical numbers for capacitors and batteries are given
below:
| device |
volumetric
energy
density
Wh/L |
power
density
W/L |
number of
charge/discharge
cycles |
discharge
time
s |
| batteries |
50-250 |
150 |
1 - 103 |
> 1000 |
| capacitors |
0.05 - 5 |
105 - 108 |
105 - 106 |
<1 |
Supercapacitors have several advantages over batteries:
they can experience virtually indefinite number of cycles (charging and
discharging), they are maintenance free, they work well in high-rate discharge,
they recharge quickly, and they have no negative environmental impact.
 v v
- Berndt, D. Maintenance-Free Batteries. New
York:: John Wiley & Sons, 1997.
- Crompton, T. R. Battery Reference Book. London:
Butterworth–Heinemann, 1990.
- Linden, D. (Ed), Handbook of Batteries.
Maidenhead: McGraw–Hill, 1995.
- Linford, R. G. (Ed), Electrochemical Science and
Technology of Polymers. New York: Elsevier, 1990.
- Ovshinsky, S. R., Fetcenko, M. A., and Ross, J. A. "A
Nickel Metal Hydride Battery for Electric Vehicles", Science 260: 1993,
176–81.
- Rechargeable Batteries Applications Handbook.
Stoneham: Butterworth–Heinemann, 1992.
- Wells, A. F. Structural Inorganic Chemistry.
Oxford: Clarendon Press, 1975.
 v
v
|
