
CHAPTER 4
CHEMICAL CHANGES IN THE BATTERY
Before explaining what happens within one storage cell, let us look into the early history of the storage battery, and see what a modest beginning the modern heavy duty battery had. Between 1850 and 1860 a man named Plante began his work on the storage battery. His original cell consisted of two plates of metallic lead immersed in dilute sulphuric acid. The acid formed a thin layer of lead sulphate on each plate which soon stopped further action on the lead. If a current was passed through the cell, the lead sulphate on the "anode" or lead plate at which the current entered the cell was changed into peroxide of lead, while the sulphate on the other lead plate or "cathode" was changed into pure lead in a spongy form. This cell was allowed to stand for several days and was then "discharged," lead sulphate being again formed on each plate. Each time this cell was charged, more "spongy" lead and peroxide of lead were formed. These are called the "active" materials, because it is by the chemical action between them and the sulphuric acid that the electricity is produced. Evidently, the more active materials the plates contained, the longer the chemical action between the acid and active materials could take place, and hence the greater the "capacity," or amount of electricity furnished by the cell. The process of charging and discharging the battery so as to increase the amount of active material, is called "forming" the plates.Plante's method of forming plates was very slow, tedious, and expensive. If the spongy lead, and peroxide of lead could be made quickly from materials which could be spread over the plates, much time and expense could be saved. It was Faure who first suggested such a plan, and gave us the "pasted" plate of today, which consists of a skeleton framework of lead, with the sponge lead and peroxide of lead filling the spaces between the "ribs" of the framework. Such plates are known as "pasted" plates, and are much lighter and more satisfactory, for automobile work than the heavy solid lead plates of Plante's. Chapter 3 describes more fully the processes of manufacturing and pasting the plates.
|
|
The foregoing description gives the final products of the chemical changes that take place in the storage battery. To understand the changes themselves requires a more detailed investigation. The substances to be considered in the chemical actions are sulphuric acid, water, pure lead, lead sulphate, and lead peroxide. With the exception of pure lead, each of these substances is a chemical compound, or composed of several elements. Thus sulphuric acid is made up of two parts of hydrogen, which is a gas; one part of sulphur, a solid, and four parts of oxygen, which is also a gas; these combine to form the acid, which is liquid, and which is for convenience written as H2SO4, H2 representing two parts of hydrogen, S one part of sulphur, and 04, four parts oxygen. Similarly, water a liquid, is made up of two parts of hydrogen and one part of oxygen, represented by the symbol H2O. Lead is not a compound, but an element whose chemical symbol is Pb, taken from the Latin name for lead. Lead sulphate is a solid, and consists of one part of lead, a solid substance, one part of sulphur, another solid substance, and four parts of oxygen, a gas. It is represented chemically by Pb SO4. Lead peroxide is also a solid, and is made up of one part of lead, and two parts of oxygen. In the chemical changes that take place, the compounds just described are to a certain extent split up into the substances of which they are composed. We thus have lead (Pb), hydrogen (H), oxygen (0), and sulphur (S), four elementary substances, two of which are solids, and two gases. The sulphur does not separate itself entirely from the substances with which it forms the compounds H2SO4 and Pb SO4. These compounds are split into H2 and SO4 and Pb and SO4 respectively. That is, the sulphur always remains combined with four parts of oxygen.Let us now consider a single storage cell made up of electrolyte, one positive plate, and one negative plate. When this cell is fully charged, or in a condition to produce a current of electricity, the positive plate is made up of peroxide of lead (PbO2), the negative plate of pure lead (Pb), and the electrolyte of dilute sulphuric acid (H2SO4). This is shown diagrammatically in Fig. 19. The chemical changes that take place when the cell is discharging and the final result of the changes are as follows:
(a). At the Positive Plate: Lead peroxide and sulphuric acid produce lead sulphate, water, and oxygen, or:
![]()
(b). At the Negative Plate: Lead and sulphuric acid produce lead sulphate and Hydrogen, or:
![]()
The oxygen of equation (a) and the hydrogen of equation (b) combine to form water, as may be shown by adding these two equations, giving one equation for the entire discharge action:
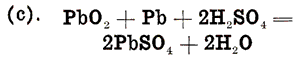
In this equation we start with the active materials and electrolyte in their original condition, and finish with the lead sulphate and water, -which are the final products of a discharge. Examining this equation, we see that the sulphuric acid of the electrolyte is used up in forming lead sulphate on both positive and negative plates, and is therefore removed from the electrolyte. This gives us the easily remembered rule for remembering discharge actions, which, though open to question from a strictly scientific viewpoint, is nevertheless convenient: |
|
Taking the cell in its discharged condition, let us now connect the cell to a generator and send current through the cell from the positive to the negative plates. This is called "charging" the cell. The lead sulphate and water will now gradually be changed back into lead, lead peroxide, and sulphuric acid. The lead sulphate which is on the negative plate is changed to pure lead; the lead sulphate on the positive plate is changed to lead peroxide, and sulphuric acid will be added to the water. The changes at the positive plate may be represented as follows:Lead sulphate and water produce. sulphuric acid, hydrogen and lead peroxide, or:
![]()
The changes at the negative plate may be expressed as follows: Lead sulphate and water produced sulphuric acid, oxygen, and lead, or:
![]()
The hydrogen (H2) produced at the positive plate, and the oxygen (0) produced at the negative plate unite to form water, as may be shown by the equation:
![]()
Equation (f) starts with lead sulphate and water, which, as shown in equation (c), are produced when a battery is discharged. It will be observed that we start with lead sulphate and water. Discharged plates may therefore be charged in water. In fact, badly discharged negatives may be charged better in water than in electrolyte. The electrolyte is poured out of the battery and distilled water poured in. The acid remaining on the separators and plates is sufficient to make the water conduct the charging current.In equation (f), the sulphate on the plates combines with water to form sulphuric acid. This gives us the rule:
During charge, acid is. driven out of the plates.This rule is a convenient one, but, of course, is not a strictly correct statement.
The changes produced by sending a current through the cell are also gradual, and will take place faster as the current is made greater. When all the lead sulphate has been 'used up by the chemical changes caused by the current, no further charging can take place. If we continue to send a current through the cell after it is fully charged, the water will continue to be split up into hydrogen and oxygen. Since, however, there is no more lead sulphate left with which the hydrogen and oxygen can combine to form lead, lead peroxide, and sulphuric acid, the hydrogen and oxygen rise to the surface of the electrolyte and escape from the cell. This is known as "gassing," and is an indication that the cell is fully charged.
Relations Between Chemical Actions and Electricity.
We know now that chemical actions in the battery produce electricity and that, on the other hand, an electric current, sent through the battery from an outside source, such as a generator, produces chemical changes in the battery. How are chemical changes and electricity related? The various chemical elements which we have in a battery are supposed to carry small charges of electricity, which, however, ordinarily neutralize one another. When a cell is discharging, however, the electrolyte, water, and active materials are separated into parts carrying negative and positive charges, and these "charges" cause what we call an electric current to flow in the apparatus attached to the battery.Similarly, when. a battery is charged, the charging current produces electrical "charges" which cause the substances in the battery to unite, due to the attraction of position and negative charges for one another. This is a brief, rough statement of the relations between chemical reactions and electricity in a battery. A more thorough study of the subject would be out of place in this book. It is sufficient for the repairman to remember that the substances in a battery carry charges of electricity which become available as an electric current when a battery discharges, and that a charging current causes electric charges to form, thereby "charging" the battery.
|
|
|
| |||||||||